Team:Heidelberg/Parts/Characterization
From 2008.igem.org


Part Characterization
Bacteriophage lambda cI (BBa_K150004)
cI protein - an introduction
Bacteriophage λ cI exists as an inactive monomer at very low concentrations (<10-9 M) but forms functional homodimers at physiological concentrations that remarkably lack a global symmetry [1]. It consists of 236 amino acids, although 237 amino acids are translated since the initiator methionine is removed by the host. Although λ cI is commonly called a repressor because of its negative regulatory functions at oL and oR, cI protein acts also as a positive regulator of gene transcription and can activate transcription of its own gene through the pRM promoter in bacteriophage λ. In genetic and biochemical studies as well as through the crystal structure it has been shown, that the carboxy-terminal domain of the cI protein contains the major sites for dimerization and oligomerization [2, 3]. The cI protein has a two-domain structure with an N-terminal portion involved in DNA binding, and a C-terminal domain that mediates dimer formation, dimer-dimer interaction, and self-cleavage. The self-cleavage reaction is triggert when the lysogenic cell suffers DNA damage and depends upon an activated form of the bacterial RecA protein [2, 4-6]. The ‘hinge region’ connecting both domains contains a conserved site that can undergo this RecA-mediated autodigestion resulting in inactivation of the repressor by separating the N-terminal from the C-terminal domain of the repressor [4, 7]. The cI protein binds symmetrically to DNA, so that each amino-terminal domain contacts a similar set of bases. The N-terminal DNA-binding domain is made up of 5 α-helices of which helix 2 and helix 3 (the helix-turn-helix motif) are involved in nucleotide sequence specific DNA recognition and binding to the major groove of DNA [8-11].
Function of cI protein in bacteriophage λ
The cI repressor in bacterophage λ is the key component of a ‘genetic switch’ that enables the phage to transition from lysogenic growth to lytic development. The cI protein binds the oL and oR operator which overlap with the pL and pR promoter (the lytic promoters). This binding allows the maintenance of the lysogenic state to be governed by cI alone. As soon as cI is inactivated, e.g., by the SOS response after UV damage or other agents that cause DNA damage, the lytic development follows [12]. The two operators oL and oR both contain three binding sites for cI protein. In each case, site 1 (i.e. oL1 and oR1) has a ~10-fold greater affinity than the other sites for cI protein. The repressor, therefore, always binds first to oL1 and oR1 and than binds to the other sites in the operator in a cooperative manner [13, 14]. The carboxy-terminal domains of the repressor dimmers mediate this cooperativity which improves the specificity and strength of the cI DNA binding, enabling strong repression of the lytic promoters [15]. Furthermore, another cooperative interaction between these two sets of tetramers bound to oL and oR, 2.4 kb apart, leads to the formation of a DNA loop held by a cI octamer, i.e. two interacting tetramers, that enhances repression of the early promoters [16-19]. In this DNA-multiprotein complex, the cI dimer bound at oR2 represses pR and at the same time also stimulates pRM transcription, thus activating cI synthesis in a repressed prophage by a positive autoregulatory loop [20-22]. As the cI concentration increases because of pRM activation, two additional cI dimers are recruited to bind oL3 and oR3 to further stabilize the oL – oR loop. In this context, cI overexpression is prevented by the binding of cI to oR3 which represses pRM [17, 19]. This positive and negative autoregulation at pRM by cI ensures a narrow range of cI repressor level to be maintained, which is optimum for stable lysogeny but is at the same time adjusted low enough for efficient induction of the lysogen. By repressing transcription from the pR promoter not only expression of genes in that operon is inhibited but also phage DNA replication by preventing transcriptional activation of λ ori, the site where phage DNA replication is initiated [23]. Even if O and P functions are present this inhibition occurs and appears to be critical for establishing a lysogen [24, 25]. The stable lysogen produces sufficient repressor not only to block prophage lytic development but also to block lytic development of any extraneous infection phage, thus imparting immunity to the lysogen against lytic superinfection [26].
Characterization of cI (BBa_K150004)
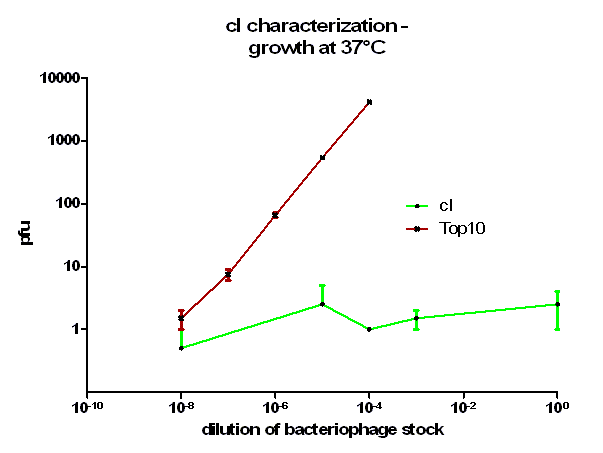
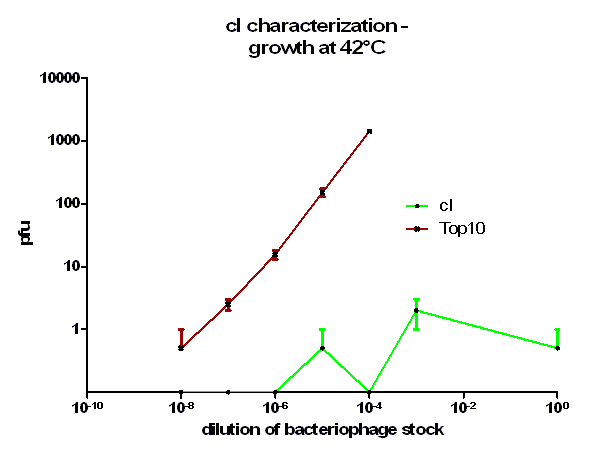
For the characterization of λ cI protein its ability to repress lytic development of bacteriophage λ was measured over a range of temperature from 28 °C to 42 °C in a phage burst experiment. If the used cI is functional no plaques should appear in a lawn of cells harbouring this cI when infected with a phage that lacks its natural cI. In control cells in contrast every of this virulent particles should lead to a plaque.
Protocol
Preparation of plating bacteria A overnight culture of the appropriate E. coli strain was grown in LB medium containing 10 mM MgSO4 and 0.2 % maltose at 30 °C to reduce the amount of cell debris in the medium. For this characterization E. coli Top10 and E. coli Top10 with transformed cI were used. The added maltose leads to a substantial induction of the maltose operon including the lamb gene, which encodes the cell surface receptor to which bacteriophage λ binds. After harvesting the cells at 3000g for 10 minutes they were resuspended in 10 mM MgSO4 and diluted to a final concentration of 2.0 OD600. The suspension of plating bacteria was stored at 4 °C for up to 1 week.
Bacteriophage λ plaque assay
Tenfold serial dilutions of the bacteriophage λ stocks were prepared. In this case a bacteriophage mutant was used lacking a functional cI which would therefore always lead to plaques. From these dilutions 100 µl were mixed with the same amount of plating bacteria, incubated for 20 minutes at 37 °C to allow the bacteriophage particles to adsorb to the bacteria, this mixture than added to 3 ml molten top agar which was kept liquid at 48 °C and the entire contend poured onto a agar plate. After harden of the top agar the inverted plates were incubated at indicated temperatures over night. On the next day plaques could be counted.
Results
As it can be seen in figure 3 – 5 the cells harbouring the part BBa_K150004 are not lysed by the used phage. This means that the constructed cI protein generator successfully expresses high levels of cI protein which is functional. Through the use of different temperatures it could be shown that the ability of the used cI protein to repress lytic development is not dependent of the temperature.

References
[1] S. Stayrook, P. Jaru-Ampornpan, J. Ni, A. Hochschild, M. Lewis, Crystal structure of the lambda repressor and a model for pairwise cooperative operator binding, Nature 452 (2008) 1022-1025.
[2] C.O. Pabo, R.T. Sauer, J.M. Sturtevant, M. Ptashne, The lambda repressor contains two domains, Proc Natl Acad Sci U S A 76 (1979) 1608-1612.
[3] C.E. Bell, P. Frescura, A. Hochschild, M. Lewis, Crystal structure of the lambda repressor C-terminal domain provides a model for cooperative operator binding, Cell 101 (2000) 801-811.
[4] J.W. Little, Autodigestion of lexA and phage lambda repressors, Proc Natl Acad Sci U S A 81 (1984) 1375-1379.
[5] R.T. Sauer, M.J. Ross, M. Ptashne, Cleavage of the lambda and P22 repressors by recA protein, J Biol Chem 257 (1982) 4458-4462.
[6] R.T. Sauer, C.O. Pabo, B.J. Meyer, M. Ptashne, K.C. Backman, Regulatory functions of the lambda repressor reside in the amino-terminal domain, Nature 279 (1979) 396-400.
[7] J.W. Little, LexA cleavage and other self-processing reactions, J Bacteriol 175 (1993) 4943-4950.
[8] C.O. Pabo, M. Lewis, The operator-binding domain of lambda repressor: structure and DNA recognition, Nature 298 (1982) 443-447.
[9] L.J. Beamer, C.O. Pabo, Refined 1.8 A crystal structure of the lambda repressor-operator complex, J Mol Biol 227 (1992) 177-196.
[10] A. Hochschild, Transcriptional activation. How lambda repressor talks to RNA polymerase, Curr Biol 4 (1994) 440-442.
[11] C.O. Pabo, R.T. Sauer, Transcription factors: structural families and principles of DNA recognition, Annu Rev Biochem 61 (1992) 1053-1095.
[12] A. Hochschild, The lambda switch: cI closes the gap in autoregulation, Curr Biol 12 (2002) R87-89.
[13] A.D. Johnson, B.J. Meyer, M. Ptashne, Interactions between DNA-bound repressors govern regulation by the lambda phage repressor, Proc Natl Acad Sci U S A 76 (1979) 5061-5065.
[14] M. Ptashne, Genetic switch: phage lambda revisited, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2004.
[15] I.B. Dodd, K.E. Shearwin, J.B. Egan, Revisited gene regulation in bacteriophage lambda, Curr Opin Genet Dev 15 (2005) 145-152.
[16] I.B. Dodd, A.J. Perkins, D. Tsemitsidis, J.B. Egan, Octamerization of lambda CI repressor is needed for effective repression of P(RM) and efficient switching from lysogeny, Genes Dev 15 (2001) 3013-3022.
[17] I.B. Dodd, K.E. Shearwin, A.J. Perkins, T. Burr, A. Hochschild, J.B. Egan, Cooperativity in long-range gene regulation by the lambda CI repressor, Genes Dev 18 (2004) 344-354.
[18] B. Revet, B. von Wilcken-Bergmann, H. Bessert, A. Barker, B. Muller-Hill, Four dimers of lambda repressor bound to two suitably spaced pairs of lambda operators form octamers and DNA loops over large distances, Curr Biol 9 (1999) 151-154.
[19] S.L. Svenningsen, N. Costantino, D.L. Court, S. Adhya, On the role of Cro in lambda prophage induction, Proc Natl Acad Sci U S A 102 (2005) 4465-4469.
[20] B.J. Meyer, M. Ptashne, Gene regulation at the right operator (OR) of bacteriophage lambda. III. lambda repressor directly activates gene transcription, J Mol Biol 139 (1980) 195-205.
[21] D. Jain, B.E. Nickels, L. Sun, A. Hochschild, S.A. Darst, Structure of a ternary transcription activation complex, Mol Cell 13 (2004) 45-53.
[22] B.E. Nickels, S.L. Dove, K.S. Murakami, S.A. Darst, A. Hochschild, Protein-protein and protein-DNA interactions of sigma70 region 4 involved in transcription activation by lambdacI, J Mol Biol 324 (2002) 17-34.
[23] M.E. Furth, W.F. Dove, B.J. Meyer, Specificity determinants for bacteriophage lambda DNA replication. III. Activation of replication in lambda ric mutants by transcription outside of ori, J Mol Biol 154 (1982) 65-83.
[24] K. Mensa-Wilmot, K. Carroll, R. McMacken, Transcriptional activation of bacteriophage lambda DNA replication in vitro: regulatory role of histone-like protein HU of Escherichia coli, EMBO J 8 (1989) 2393-2402.
[25] M.S. Wold, J.B. Mallory, J.D. Roberts, J.H. LeBowitz, R. McMacken, Initiation of bacteriophage lambda DNA replication in vitro with purified lambda replication proteins, Proc Natl Acad Sci U S A 79 (1982) 6176-6180.
[26] D.L. Court, A.B. Oppenheim, S.L. Adhya, A new look at bacteriophage lambda genetic networks, J Bacteriol 189 (2007) 298-304.
[27] C.E. Bell, M. Lewis, Crystal structure of the lambda repressor C-terminal domain octamer, J Mol Biol 314 (2001) 1127-1136.
oriT R (BBa_J01003)
We have characterized the oriT BBa_J01003 in a conjugation system in which pUB307 is used as helper plasmid and E. coli as donor and recipient cells. We measured quantitatively the conjugative competence of oriT BBa_J01003 by conjugation between different strains, at different temperatures and with different donor/recipient ratios. We also compared the conjugation kinetics of BBa_J01003 and pUB307 quantitatively.
The part oriT R BBa_J01003 was cotransformed together with pUB307 (helper plasmid) into E. coli strain Top10. These cells therefore became conjugation donor and have a kanamycin (from pUB307) and ampicillin (from BBa_J01003) resistance. The recipient cells have the plasmid pBAD33 with a chloramphenicol resistance. After mixing both cells types the conjugation starts and BBa_J01003 as well as pUB307 can be transported. The helper plasmid pUB307 can still be transported since it still contains its oriT region. The transconjugants which have received BBa_J01003 through the conjugation have ampicillin and chloramphenicol resistance and can therefore be easily selected.
Test 1 Conjugation kinetics of oriT BBa_J01003 between E. coli Top10
On the first day we inoculated LB + antibiotics medium with a single colony or from a glycerol stock. For this test we used donor and the recipient cells that were both E. coli Top10. On the second day we washed the cells of the overnight culture twice with LB medium, transferred the donor cell suspension to the pellet of the recipient cells and resuspended the recipient cells in this medium. After another centrifugation step we resuspended the pellet which now is a mixture of donor and recipient cells in 100 µl LB medium without antibiotics. We than transferred the cell suspension on a membrane filter, which was placed on LB plates before and incubated these plates at 37 °C for 0, 6, 12, 18, 24, 30, 36, 42 minutes. Afterwards the membrane was put into 1 ml LB medium and the cells resuspended by vortexing for 30 seconds. The cell suspension was diluted 10-5 and plated on the LB / ampicillin + chloramphenicol (CA) plates. The plates were incubated at 37 °C overnight and on the third day the colonies could be counted. We have done this test thrice independently and calculated the conjugation efficient which is defined as [transconjugants per ml] / [donor per ml] from this assay. To count the donor and recipient cell density small aliquots of the used suspensions were diluted 10-7 and plated on agar plates containing kanamycin + ampicillin for donor cells and chloramphenicol for the recipients.
Table 1
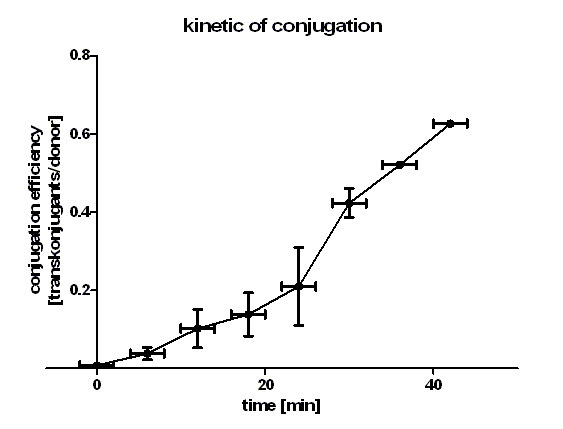
Figure 1 shows that the kinetic of the conjugative plasmid BBa_J01003 has three phases. In the first (0 min to 24 min) and in the third phase (30 min to 42 min) the curve has a smaller slope, whereas the second phase (24 min to 30 min) it has a very big slope. An explanation for this result could be that the cell growth in the first 24 min can be neglected due to the average generation time of E. coli in the LB medium which lies between 20 and 30 minutes whereas in the second phase the growth of E. coli dominates the conjugation process and leads to this deviation from linearity. In this test we used very concentrated bacteria suspension in a small conjugation volume which leads to the observation of conjugation in an approximately linear process in the primary phase. We have done a linear regression analysis for the first phase (0 min to 24 min) and we calculated the slope for this phase as 0.008397 [transconjugants / donor / min]. We calculated the conjugation rate for BBa_J01003 which is defined as transconjugants / donor / recipient / minutes. The conjugation rate of BBa_J01003 is 1.05 • 10-12 [ml • min-1 • cell-1]. This rate was used by the modeling group as a parameter value. Figure 1 also shows us that the transport of the plasmid pSB1A2 containing the part BBa_J01003 by conjugation is a very fast process since we got transconjugants in the first minutes of the experiment. Through this experiment we can conclude that the transport of the plasmid pSB1A2 containing BBa_J01003 by conjugation needs only about 2 minutes. Since we have two conjugative plasmids in our donor cells, we wanted to see which plasmid is preferred to be transported by conjugation. We therefore plated the cells in the test C (see table 2) also on agar plates containing chloramphenicol + kanamycin (CK) and chloramphenicol + kanamycin + ampicillin (CKA) to select for transconjugants getting pUB307 and both plasmids.
Table 2

Figure 2 clearly shows that the plasmid pSB1A2 containing only BBa_J01003 is preferably transported during the first 20 minutes in comparison to pUB307. Since pUB307 has the same oriT as BBa_J01003 is, we assume the size of the plasmid to be the reason for this since BBa_J01003 in pSB1A2 is approx. 2.5 kb in size whereas pUB307 has 60 kb. A comparison of the red and the green curve shows us that the difference in the duration of the transport of plasmids with different size is not very big. Looking at the blue curve you can see that the first transconjugants with both plasmids can be detected after approx. 18 minutes which is longer than the sum of both transport times.
Test 2 Conjugation kinetics of oriT BBa_J01003 between different E.coli strains
For this experiment we used the same technique as described in test 1. In this case we used different E. coli strains as recipients, namely E. coli Top10, MG1655 and DH5α. In this experiment the cells on the membranes were incubated for 0, 6, 12, 18, 24, 30, 36 and 42 minutes at 37 °C. This test was conducted twice independently and the conjugation efficiency calculated as described in test 1.
Table 3
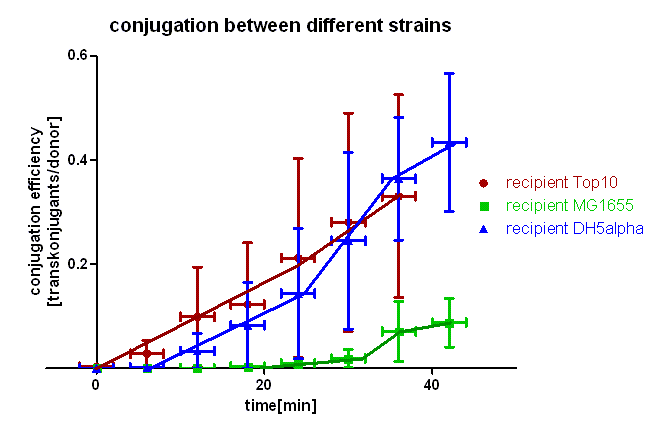
As you can see in figure 3 there is a difference in the conjugation kinetic between Top10 as donor and different strains as recipient. With E. coli Top10 as donor E. coli DH5α is a comparably good recipient as Top10 whereas MG1655 showed up as a bad recipient under these conditions. Comparing the red with the blue curve one can see that DH5α transconjugants appear later (6 minutes) that Top10, but the conjugation efficiency and also the conjugation rate (slope between 6 and 24 minutes) are in the same range with Top10 and DH5α. We assume that more time is needed for stabilizing the pillus contact between Top10 and DH5α than between two Top10 cells, but the conjugation can be done between Top10 and DH5α as good as between two Top10 cells. Comparing the red and the green curve one can see that the first MG1655 transconjugants appear much later (20 minutes) and the conjugation efficiency and rate (slope between 20 and 32 minutes) are much smaller. Therefore we conclude that conjugation between different strains is possible using this oriT but the efficiency depends on the used strains and their compatibility.
Test 3 Conjugative competence of oriT BBa_J01003 at different donor/recipient ratios
In this experiment we again used the same protocol as described above in test 1 but used different donor / recipient rations, namely 50:1, 25:1, 10:1, 8:1, 5:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:5, 1:10, 1:25, and 1:50. This test was conducted twice independently and the conjugation efficiency calculated as described above. The donor and recipient cell density was measured by plating 10-7 dilution on agar plates containing kanamycin + ampicillin for the donor and chloramphenicol for recipient cells.
Table 4
Figure 4 shows very clearly that at same cell density, if donor : recipient ratio is 1 : 2 the highest amount of transconjugants can be achieved. A big difference in the amount of donor : recipient leads to a low number of transconjugants. At low donor : recipient ratios the conjugation efficiency was quiet high but lead to a low number of transconjugants since the density of donor cells was too low. Only at donor : recipient rations between 1 : 1 and 1 : 3, a high conjugation efficiency and a high donor density was achieved leading to a high number of transconjugants. A maximum in the number of transconjugants could be achieved at a ratio of 1 : 2.
Test 4 Conjugative competence of oriT BBa_J01003 at different temperatures
In this experiment the same method is used as described above in test 1. Here, donor and recipient both were E. coli Top10. The incubation during conjugation took place at different temperatures, namely 4 °C, 22 °C, 30 °C, 37 °C and 42°C for 20, 40 and 60 minutes. The cell suspension was diluted 10-6 and plated on agar containing ampicillin and chloramphenicol (CA). This experiment was conducted twice independently. The donor and recipient cell density was again calculated by plating 10-7 dilutions on plates containing kanamycin and ampicillin or chloramphenicol, respectively.
Table 5
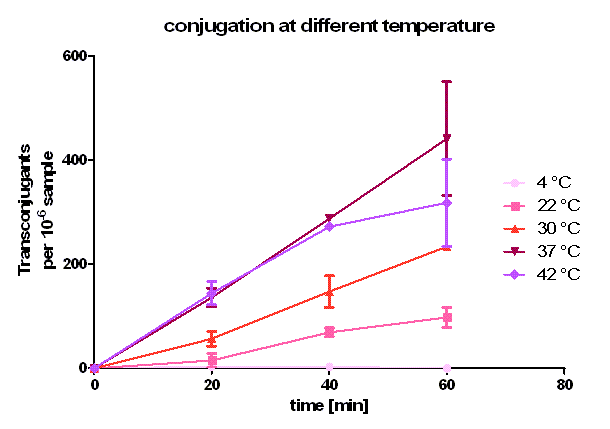
As you can see in figure 5, conjugation is most efficient at 37 °C. From 0 to 20 minutes during the primary conjugation phase the cell growth can be neglected. From 20 to 40 minutes, the cell growth is also presented in the conjugation curves. At 4 °C, the conjugation curve has a very small slope (near zero) and no significant transconjugants production could be observed. At 22, 30, 37 and 42 °C, a significant number of transconjugants could be achieved, but the conjugation efficiency and the conjugation rate (slope between 0 and 20 minutes) differ strongly. From 22 °C to 37°C, the conjugation efficiency and conjugation rate increases with rising temperature. Comparing these three curves between 20 and 40 minutes one can see that the cell growth gets more important with rising temperature. Dynamically, the cells have more mobility at higher temperature, and have a bigger possibility to touch another cell, which is necessary for conjugation. When we compared the conjugation curve at 42 °C with the one at 37 °C, one can see, that the conjugation efficient and the conjugation rate at 42 °C is as good as at 37 °C. After the primary conjugation phase, the production of transconjugants at 42 °C is clearly worth than the production at 37 °C what can be seen at comparing the curves between 40 and 60 minutes. A possible reason for this could be a lower growth and higher death rate at 42 °C compared to 37 °C. So, one can conclude, that for best conjugation efficiency one need a warm environment for the conjugation to take place but also temperatures which favor cell growth.
Summary
The characterization of BBa_J01003 in pSB1A2 has given the following results:
- The conjugation has an average conjugation rate of 1.05 • 10-12 [ml • min-1 • cell-1]
- The conjugation has an approximate transport time of 2 minutes
- The oriT BBa_J01003 will be transported first in attendance of pUB307 in the donor cells
- Smaller plasmids favor the conjugation and are conjugated first
- The oriT BBa_J01003 can be conjugated between Top10 cells, from Top10 to DH5α cells with similar efficiency but from Top10 to MG1655 with a strongly lower efficiency
- The conjugation favors at the donor : recipient ratio of 1 : 2
- The conjugation has the best production of transconjugants, the best efficiency and the best conjugation rate at 37 °C
LuxS from Vibrio harveyi (partly characterized)
LuxS encodes for the protein (LuxS autoinducer synthase) that is responsible for the synthesis of autoinducer 2 (AI-2) in many species of Gram-negative and Gram-positive bacteria. This LuxS construct is cloned from Vibrio harveyi, a Gram-negative bioluminescent marine bacterium, which regulates light production in response to two distinct autoinducers (AI-1 and AI-2). [1]
Functional check of LuxS/AI-2 production
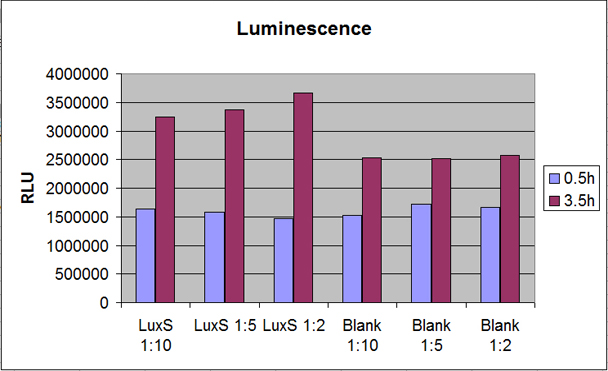
The functionality of the new LuxS part that was cloned by PCR from V. harveyi (BB120) in a ptrc99α plasmid was proven by measurement of luciferase activity in Vibrio as a reporter strain. DH5α cells with the new construct pTrc99α_LuxS were grown up and the supernatant was harvested by using centrifugation and sterile filtration. The gained supernatant was added to the reporter cells in different dilutions and incubated for three hours. Luminescence and OD were measured after 0.5h and 3,5h.
Results
Cell growth rate of all samples LuxS and Blank was shown to be similar in OD measurements (data not shown). Luminescence of Vibrio reporter strain (data shown in figure 1) seems to increase with the concentration of supernatant in the cell delusion. Blanks with supernatant of DH5α cells transformed with pTrc99α plasmid show a significantly smaller Luminescence and there is no increase of Luminescence for increasing supernatant concentration in the cell delusion.
Reference
[1] The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001 Jul; 41(2):463-76.
 "
"