Team:BrownTwo/Limiter/utility
From 2008.igem.org
The utility of threshold limitationMuch as excessive signal levels can destroy a radio transmitter, excessive genetic expression can cause damage in living systems. While cells are usually well-equipped to modulate their own transcriptional behavior, an investigation into pathological gene expression indicates that many situations arise wherein the expected regulatory response fails. Our device is designed to augment endogenous gene regulatory pathways.
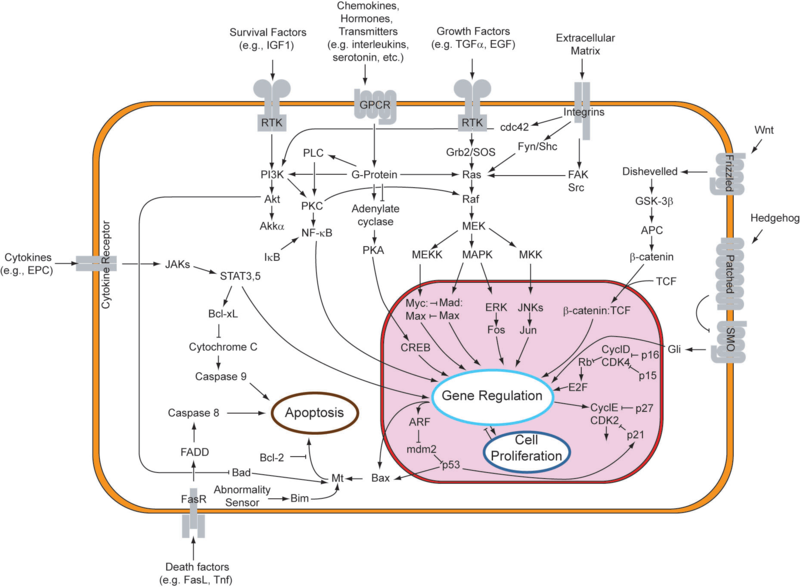
While one can surely identify numerous cases of abnormal gene expression in cellular systems, we turn our attention to one particular network related to a crucial decision in cell fate, apoptosis. Cellular suicide and the cancer phenotypeIn response to either stressful circumstances or communication from neighbors, cells will often undergo a highly regulated process that results in cell death, known as apoptosis. Pro-apoptotic and anti-apoptotic factors expressed in a viable cell strike an ongoing balance that codes for cell survival. Such a balance tilts in favor of an apoptotic cell fate in the presence of appropriate stimuli, like oxidative stress or UV treatment. In multicellular organisms, programmed cell death plays a healthy role in development and disease regulation. One might say that the system of apoptosis evolved to provide individual cells a method of succumbing to the greater good of the whole organism. As can be observed in the figure below, the genetic underpinnings that encode for apoptosis are truly immense, and are intricately linked to those for cell proliferation. An apoptotic response generally entails changes to multiple nodes in this robust network of genes. One important point to note is that apoptosis can be triggered extrinsically, from factors in the cell's environment, or intrinsically, when the cell recognizes that it should not continue to proliferate. Due to its complexity, the system is still being explored, especially for its potential involvement in cancer. Given the function of apoptosis in defining cell fate, it should come as no surprise that multiple disease states are characterized by an inactive or reduced apoptotic response. When apoptosis cannot proceed, often due to mutations in the genes responsible for initiating the pathway, cell proliferation can occur unchecked. This allows for propagation of mutated DNA as well as an abnormal accumulation of cells. This phenotype is highly characteristic of tumor formation in multicellular organisms. In designing a system to target abnormally-absent apoptosis, one can consider two approaches. It is possible to identify a key anti-apoptotic factor that requires down-regulation or, conversely, to target a pro-apoptotic factor that requires up-regulation. For the former, one might consider X-linked Inhibitor of Apoptosis (XIAP), which, while not present on the above figure, is responsible for inhibiting caspases (Vucic and Fairbrother, 2007). Caspase activation in the apoptotic pathway can be considered to be a point of no return, a trigger for the irreversible decision to undergo cellular suicide. Downregulation of XIAP should thus help initiate apoptosis. Alternatively, mutations in the gene encoding p53 have been found to exist in 50% of cancers (Toledo and Wahl, 2006). These mutations occur overwhelmingly in the DNA binding domain of this protein, thus disabling its ability as a transcriptional regulator. Since the inhibition of apoptosis stems from a defective p53, it might be necessary to introduce a protein into the cell with analogous activity to p53. Upregulation of this exogenous genetic input would then result in apoptosis. In either situation, it is essential to keep in mind that the maintenance of factors that promote and inhibit apoptosis defines a healthy cell. Any regulatory system set in place must respond appropriately to apoptotic stimuli, and should otherwise exist passively in the system when no regulation is needed. These considerations emphasize the need for a limiter-type regulatory device.
Apoptosis in yeastWhile still an ongoing debate, there is increasingly more evidence to suggest that yeast have apoptosis as well (Frohlich et al., 2007). Despite the fact that apoptosis seems to make most sense in a multicellular context, the organisms nonetheless show signs characteristic of a programmed cell death. In fact, much research about apoptotic pathways from mammals can be conducted in yeast, due to the fact that many of the genes and pathways present in mammals share homology to those found in yeast. Given this promising range of homology, one might consider first applying the regulatory approach discussed above for mammals in a yeast framework. A limiter device in yeast could also serve as a useful tool for understanding important nodes in the apoptotic network. References
|
 "
"