Team:Chiba/Calendar-Home/21 August 2008
From 2008.igem.org
(Difference between revisions)
(→Team:Communication) |
(→Team:Communication) |
||
| (20 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
==Laboratory work== | ==Laboratory work== | ||
===Team:Input=== | ===Team:Input=== | ||
| - | (-->20/8) | + | (-->20/8) |
| + | |||
| + | Inoculated transformants for 12 hours in LB 2mL containing Ampicillin. | ||
*[http://partsregistry.org/Part:BBa_R0051 BBa_R0051](2007) | *[http://partsregistry.org/Part:BBa_R0051 BBa_R0051](2007) | ||
| Line 14: | Line 16: | ||
*[http://partsregistry.org/Part:BBa_J22141 BBa_J22141](2007) | *[http://partsregistry.org/Part:BBa_J22141 BBa_J22141](2007) | ||
| - | + | BBa_J22141:After inoculated for 11 hours, added 20μl IPTG and cultured one hour. | |
| - | + | ||
| - | * | + | *UV irradiation test (plate phase) |
| - | *two plasmids from | + | *two plasmids from(JW1908) |
**(Repressor)-Ptrc-LuxR-Plux-cI-colE1-Amp- | **(Repressor)-Ptrc-LuxR-Plux-cI-colE1-Amp- | ||
| - | **(Reporter)-PcI-GFP-p15a-Cm-( | + | **(Reporter)-PcI-GFP-p15a-Cm- |
| + | |||
| + | #Inoculated (Reporter) culture from glycerol stock(-80°C freezer) in 2mL LB-Ampicillin, Chloramphenicol medium and LB-Ampicillin, Chloramphenicol, 100nmAHL mediumfor 12h, at 37°C. | ||
| + | #We diluted 10<sup>5</sup>-fold, and plated 20ul of the resulting solution to (LB-Amp,Cm)(LB-Amp,cm,AHL100nM) plates each other. | ||
| + | #We cultured for 12h at 37°C. | ||
| + | #We iraddiated UV each plate. Wavelength:254nm,distance from the UV lamp to the cultures were 14cm. | ||
| + | #We performed the same operations on plates following 9 or 21h of UV | ||
| + | exposure. | ||
| + | #Colonies from both plates were picked and cultured in 2mL of | ||
| + | LB-Amp,Cm or LB-Amp, Cm, 100nMAHL for 12h at 37°C. | ||
| - | # | + | #We diluted 10<sup>5</sup>-fold, and plated 20ul of the |
| - | + | resulting solution to (LB-Amp,Cm)(LB-Amp,cm,AHL100nM) plates each other. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| Line 34: | Line 40: | ||
<table width="200" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | <table width="200" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
<td width="257"></td> | <td width="257"></td> | ||
| - | <td> | + | <td>no AHL</td><td>AHL100nM</td> |
<tr> | <tr> | ||
<td>9h</td> | <td>9h</td> | ||
| Line 44: | Line 50: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | + | result | |
| - | + | <BR> | |
| - | + | GFP fluorescence was observed from plates without AHL. | |
===Team:Communication=== | ===Team:Communication=== | ||
| Line 63: | Line 69: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dNTP mix</td> | + | <td>dNTP mix(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Foward Primer</td> | + | <td>Foward Primer(μL)</td> |
<td>5</td> | <td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Reverse Primer</td> | + | <td>Reverse Primer(μL)</td> |
<td>5</td> | <td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA polymerase TAQ</td> | + | <td>DNA polymerase TAQ(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Thermopol Buffer</td> | + | <td>Thermopol Buffer(μL)</td> |
<td>5</td> | <td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>28</td> | <td>28</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
| - | <td> | + | <td>50</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| - | : | + | :95°C,5min -> ( 95°C,1min -> 52°C,1min -> 72°C,1min )・・・25cycles -> 72°C,10min -> 6°C |
| Line 107: | Line 113: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Loading Dye</td> | + | <td>Loading Dye(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>7</td> | <td>7</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
| - | <td> | + | <td>12</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 146: | Line 152: | ||
===Team:Output=== | ===Team:Output=== | ||
[[Team:Chiba/protocol/transformation|Transformation]] | [[Team:Chiba/protocol/transformation|Transformation]] | ||
| - | *[http://partsregistry.org/Part:BBa_J32007 BBa_J32007]( | + | *[http://partsregistry.org/Part:BBa_J32007 BBa_J32007](2007)-->no colony-->no colony(2 times)-->colony(3times) |
| - | *[http://partsregistry.org/Part:BBa_B0034 BBa_B0034]( | + | *[http://partsregistry.org/Part:BBa_B0034 BBa_B0034](2007)-->colony |
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 05:58, 30 October 2008
20 August 2008 <|> 22 August 2008
Contents |
Laboratory work
Team:Input
(-->20/8)
Inoculated transformants for 12 hours in LB 2mL containing Ampicillin.
- BBa_R0051(2007)
- BBa_R0051(2006)
- BBa_J06650(2007)
- BBa_J06650(2006)
- BBa_J22136(2007)
- BBa_J22141(2007)
BBa_J22141:After inoculated for 11 hours, added 20μl IPTG and cultured one hour.
- UV irradiation test (plate phase)
- two plasmids from(JW1908)
- (Repressor)-Ptrc-LuxR-Plux-cI-colE1-Amp-
- (Reporter)-PcI-GFP-p15a-Cm-
- Inoculated (Reporter) culture from glycerol stock(-80°C freezer) in 2mL LB-Ampicillin, Chloramphenicol medium and LB-Ampicillin, Chloramphenicol, 100nmAHL mediumfor 12h, at 37°C.
- We diluted 105-fold, and plated 20ul of the resulting solution to (LB-Amp,Cm)(LB-Amp,cm,AHL100nM) plates each other.
- We cultured for 12h at 37°C.
- We iraddiated UV each plate. Wavelength:254nm,distance from the UV lamp to the cultures were 14cm.
- We performed the same operations on plates following 9 or 21h of UV
exposure.
- Colonies from both plates were picked and cultured in 2mL of
LB-Amp,Cm or LB-Amp, Cm, 100nMAHL for 12h at 37°C.
- We diluted 105-fold, and plated 20ul of the
resulting solution to (LB-Amp,Cm)(LB-Amp,cm,AHL100nM) plates each other.
- Colony count
| no AHL | AHL100nM | |
| 9h | 606 | 453 |
| 12h | 146 | 151 |
result
GFP fluorescence was observed from plates without AHL.
Team:Communication
| DNA Template | 1 |
| dNTP mix(μL) | 10 |
| Foward Primer(μL) | 5 |
| Reverse Primer(μL) | 5 |
| DNA polymerase TAQ(μL) | 1 |
| Thermopol Buffer(μL) | 5 |
| dH2O(μL) | 28 |
| TOTAL(μL) | 50 |
- 95°C,5min -> ( 95°C,1min -> 52°C,1min -> 72°C,1min )・・・25cycles -> 72°C,10min -> 6°C
- --->Gel Check
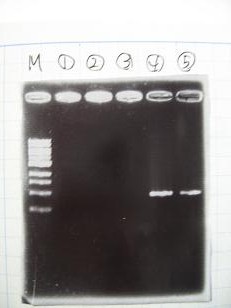
|
- Transformation
- competent cells : XL10G
- BBa_S03154(2007)
- BBa_S03154(2006)
- BBa_I9026(2007)
- BBa_I9026(2006)
- BBa_I9030(2007)
- BBa_I9030(2006)
--->(23/8)Digestion
Team:Output
- BBa_J32007(2007)-->no colony-->no colony(2 times)-->colony(3times)
- BBa_B0034(2007)-->colony
 "
"