Team:Chiba/Project/Experiments:Sender Crosstalk
From 2008.igem.org
(→Design) |
|||
| (22 intermediate revisions not shown) | |||
| Line 11: | Line 11: | ||
|} | |} | ||
__NOTOC__ | __NOTOC__ | ||
| - | |||
==Sender Crosstalk== | ==Sender Crosstalk== | ||
=== Design=== | === Design=== | ||
| Line 18: | Line 17: | ||
[[Image:AHL variety chiba.gif|frame|left|'''Fig. 2 AHL varieties''']] | [[Image:AHL variety chiba.gif|frame|left|'''Fig. 2 AHL varieties''']] | ||
| - | Each species has their own LuxI-type proteins,which synthesize their specific autoinducers, AHLs. AHLs produced by different LuxI-type proteins differ only in the length of the acyl-chain moiety and substitution at position C-3.LuxR,which is original for Vibrio fischeri, is activated by its cognate autoinducer, 3OC6HSL. However, LuxR is also activated by non-endogenous molecules, C4HSL, C6HSL, and 3OC12HSL. Activation by non-endogenous molecules requires a higher signal concentration [Team:Chiba/Project/Experiments:Sender Crosstalk# | + | Each species has their own LuxI-type proteins,which synthesize their specific autoinducers, AHLs. AHLs produced by different LuxI-type proteins differ only in the length of the acyl-chain moiety and substitution at position C-3[[Team:Chiba/Project/Experiments:Sender Crosstalk#references|<sup>(4)</sup>]].LuxR,which is original for Vibrio fischeri, is activated by its cognate autoinducer, 3OC6HSL. However, LuxR is also activated by non-endogenous molecules, C4HSL, C6HSL, and 3OC12HSL. Activation by non-endogenous molecules requires a higher signal concentration [[Team:Chiba/Project/Experiments:Sender Crosstalk#references|<sup>(1),(2)</sup>]]. This results in slower activation of receivers, when AHL concentration is increasing. |
| Line 57: | Line 56: | ||
To characterize quorum sensing crosstalk, constitutive AHL senders were mixed with constitutive receivers and measure fluorescence intensity. | To characterize quorum sensing crosstalk, constitutive AHL senders were mixed with constitutive receivers and measure fluorescence intensity. | ||
| - | #Transformed Senders into ''E. coli'' strains (XL10Gold) and Receiver into ''E. coli'' strain (JW1908). | + | #Transformed Senders into ''E. coli'' strains (XL10Gold or JW1908) and Receiver into ''E. coli'' strain (JW1908). |
| - | #Inoculated them independently in liquid media. Incubated at | + | #Inoculated them independently in liquid media. Incubated at 37°C for 12hours. |
#Mixed them. | #Mixed them. | ||
| - | #Incubated at 30°C. | + | #Incubated at 37°C or 30°C. |
#Measured intensity of green fluorescence (485 nm (excitation) and 527 nm (emission)) at regular time intervals. | #Measured intensity of green fluorescence (485 nm (excitation) and 527 nm (emission)) at regular time intervals. | ||
| Line 67: | Line 66: | ||
===Results & Discussion=== | ===Results & Discussion=== | ||
[[image:senders_crosstalk_chiba_01.gif|frame| | [[image:senders_crosstalk_chiba_01.gif|frame| | ||
| - | '''Fig. 3 senders crosstalk test.'''senders strain XL10Gold,Receiver strain JW1908.Reaction temparature was | + | '''Fig. 3 senders crosstalk test.'''senders strain XL10Gold,Receiver strain JW1908.Reaction temparature was 30°C.All measurements are averages from three replicate cultures with error bars representing standard deviations.Labeling:LuxI, RhlI, and LasI means fluorescence induced by AHLs synthesized by [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084008 BBa_K084008], and [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] respectively.]] |
| - | ====[[Team:Chiba/Sender experiments/Senders(XL10Gold) T9002(JW1908)#Reaction temparature: | + | ====[[Team:Chiba/Sender experiments/Senders(XL10Gold) T9002(JW1908)#Reaction temparature:30°C|Senders(XL10Gold), T9002(JW1908)@30°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000]]==== |
| - | + | When incubated at 30°C,E.coli strain(XL10Gold) transformed with the LasI genes produced more AHL than at 37°C. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | (Green fluorescence intensity after incubating for 8 hours was: | |
| - | + | ::163 at 37°C. | |
| - | + | ::245 at 30°C. | |
| - | + | 3OC12HSL produced by LasI protein(expressed by[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and 3OC6HSL produced by LuxI protein(expressed by[http://partsregistry.org/Part:BBa_K084012 BBa_K0840012]) both activated LuxR protein and cousese gfp expression.The increase of green fluorescence intensity caused by 3OC12HSL was more gradualy than that caused by 3OC6HSL,time before fluorescence intensity reached at 200 was 2.5 hours longer than that of the 3OC6HSL. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===Future plans=== | ===Future plans=== | ||
| Line 100: | Line 81: | ||
*To make the slope of transfer curve of our device to be steep: | *To make the slope of transfer curve of our device to be steep: | ||
| - | #Introduce Positive Feedback Loop into our | + | #Introduce Positive Feedback Loop into our genetic circuit. |
===Other Experiments=== | ===Other Experiments=== | ||
====Visual Judgement==== | ====Visual Judgement==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | fluorescence intensity was from 150 to 200 when the 2 mL of reaction culture in a test tube. | |
| - | ====[[Team:Chiba/Sender experiments/Senders( | + | ====[[Team:Chiba/Sender experiments/Senders(JW1908) T9002(JW1908)#Reaction temparature:37°C|Senders(JW1908), T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000]]==== |
| - | ====[[Team:Chiba/Sender experiments/Senders(XL10Gold) T9002(JW1908)#Reaction temparature: | + | ====[[Team:Chiba/Sender experiments/Senders(JW1908) T9002(JW1908)#Reaction temparature:30°C|Senders(JW1908),T9002(JW1908)@30°C,Sender(μL):Receiver(μL)=500:500,100:1000,10:1000]]==== |
| + | |||
| + | ====[[Team:Chiba/Sender experiments/Senders(XL10Gold) T9002(JW1908)#Reaction temparature:37°C|Senders(XL10Gold),T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500,100:1000,10:1000]]==== | ||
| + | |||
| + | ====[[Team:Chiba/Sender experiments/Senders(XL10Gold) T9002(JW1908)#Reaction temparature:25°C|Senders(XL10Gold), T9002(JW1908)@25°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000]]==== | ||
=== Demo ~Senders~ === | === Demo ~Senders~ === | ||
We experimentally tested the sender genes LuxI and LasI which produce the greatest time difference. | We experimentally tested the sender genes LuxI and LasI which produce the greatest time difference. | ||
| - | We mixed bacteria ( | + | We mixed bacteria (XL10Gold) transformed with either the LuxI or LasI genes and bacteria (of the strain JW1908) transformed with the LuxR gene at a 1:1 ratio and visually observed GFP fluorescence. |
=== Results === | === Results === | ||
| - | [[Image:Team-Chiba-demo-mihon.gif|200px|frame|left|'''Fig. 4 Demo''']]<br> Green region: sender=LuxI (50 uL), Red circular region: sender | + | [[Image:Team-Chiba-demo-mihon.gif|200px|frame|left|'''Fig. 4 Demo''']]<br> Green region: sender=LuxI (50 uL), Red circular region:sender culture containing [http://partsregistry.org/Part:BBa_K084007 LasI gene](50 μL).Receiver culture containing [http://partsregistry.org/Part:BBa_T9002 LuxR-plux-gfp gene] (50 μL) |
<gallery> | <gallery> | ||
| - | Image:Team-Chiba-demo-1.JPG|0 | + | Image:Team-Chiba-demo-1.JPG|0 hours |
| - | Image:Team-Chiba-demo-2.JPG|4 | + | Image:Team-Chiba-demo-2.JPG|4 hours <br>(LuxI GFP detected) |
| - | Image:Team-Chiba-demo-3.JPG|8 | + | Image:Team-Chiba-demo-3.JPG|8 hours <br>(LuxI GFP and LasI GFP detected) |
| - | </gallery> | + | </gallery>Fig. 5 |
| - | + | ||
-->more about [[Team:Chiba/Demo_experiments:Senders|Demo experiments detail]] | -->more about [[Team:Chiba/Demo_experiments:Senders|Demo experiments detail]] | ||
| Line 137: | Line 116: | ||
#[http://partsregistry.org/Part:BBa_F2620:Specificity BBa_F2620:Specificity] | #[http://partsregistry.org/Part:BBa_F2620:Specificity BBa_F2620:Specificity] | ||
#[http://mic.sgmjournals.org/cgi/content/full/153/12/3923 Paul Williams.:Quorum sensing, communication and cross-kingdom signalling in the bacterial world.Microbiology 153 (2007), 3923-3938] | #[http://mic.sgmjournals.org/cgi/content/full/153/12/3923 Paul Williams.:Quorum sensing, communication and cross-kingdom signalling in the bacterial world.Microbiology 153 (2007), 3923-3938] | ||
| + | #[http://www.pnas.org/content/100/suppl.2/14549.full Michiko E. Taga. Bonnie L.Bassler.:Chemical communication among bacteria.PNAS.November 25, 2003,'''100'''.suppl.2] | ||
Latest revision as of 11:00, 30 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
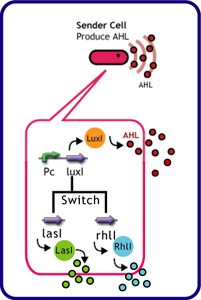
Sender Crosstalk
Design
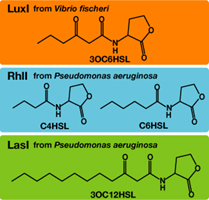
Each species has their own LuxI-type proteins,which synthesize their specific autoinducers, AHLs. AHLs produced by different LuxI-type proteins differ only in the length of the acyl-chain moiety and substitution at position C-3(4).LuxR,which is original for Vibrio fischeri, is activated by its cognate autoinducer, 3OC6HSL. However, LuxR is also activated by non-endogenous molecules, C4HSL, C6HSL, and 3OC12HSL. Activation by non-endogenous molecules requires a higher signal concentration (1),(2). This results in slower activation of receivers, when AHL concentration is increasing.
Experiments
The purpose of this experiment was to create delays in communication time using cross-talk between non-specific signals. We used the following genes for this experiment.
| senders (cell: XL10-Gold or JW1908) | receiver (cell: JW1908) |
| *plac+rbs+LasI (pSB1AK3) | *BBa_T9002 (Express GFP in response to AHL) (pSB1A3) |
| *plac+rbs+RhlI (pSB1AK3) | |
| *BBa_K084009 | |
| *plac+rbs+LuxI (pSB1AK3) | |
| *BBa_K084014 | |
| *BBa_S03623(ptet+rbs+LuxI(LVA)) |
Details
Method
To characterize quorum sensing crosstalk, constitutive AHL senders were mixed with constitutive receivers and measure fluorescence intensity.
- Transformed Senders into E. coli strains (XL10Gold or JW1908) and Receiver into E. coli strain (JW1908).
- Inoculated them independently in liquid media. Incubated at 37°C for 12hours.
- Mixed them.
- Incubated at 37°C or 30°C.
- Measured intensity of green fluorescence (485 nm (excitation) and 527 nm (emission)) at regular time intervals.
Results & Discussion
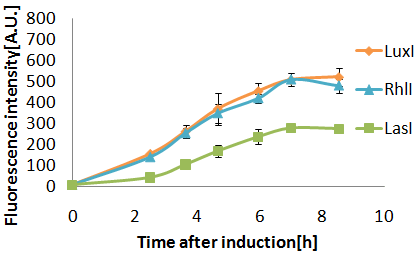
Senders(XL10Gold), T9002(JW1908)@30°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
When incubated at 30°C,E.coli strain(XL10Gold) transformed with the LasI genes produced more AHL than at 37°C.
(Green fluorescence intensity after incubating for 8 hours was:
- 163 at 37°C.
- 245 at 30°C.
3OC12HSL produced by LasI protein(expressed byBBa_K084007 and 3OC6HSL produced by LuxI protein(expressed byBBa_K0840012) both activated LuxR protein and cousese gfp expression.The increase of green fluorescence intensity caused by 3OC12HSL was more gradualy than that caused by 3OC6HSL,time before fluorescence intensity reached at 200 was 2.5 hours longer than that of the 3OC6HSL.
Future plans
- Reduce quantity of expression of an enzyme composing AHL
- Replace medium RBS with weak one.
- Alter the copy number of plasmids in the sender into low.
- To make the slope of transfer curve of our device to be steep:
- Introduce Positive Feedback Loop into our genetic circuit.
Other Experiments
Visual Judgement
fluorescence intensity was from 150 to 200 when the 2 mL of reaction culture in a test tube.
Senders(JW1908), T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
Senders(JW1908),T9002(JW1908)@30°C,Sender(μL):Receiver(μL)=500:500,100:1000,10:1000
Senders(XL10Gold),T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500,100:1000,10:1000
Senders(XL10Gold), T9002(JW1908)@25°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
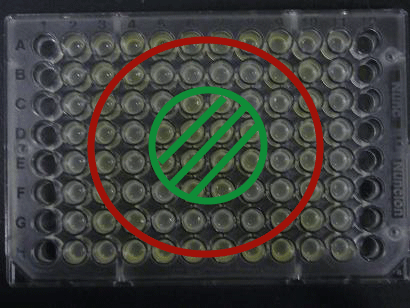
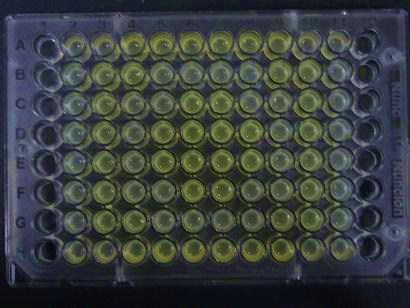
Demo ~Senders~
We experimentally tested the sender genes LuxI and LasI which produce the greatest time difference. We mixed bacteria (XL10Gold) transformed with either the LuxI or LasI genes and bacteria (of the strain JW1908) transformed with the LuxR gene at a 1:1 ratio and visually observed GFP fluorescence.
Results
Green region: sender=LuxI (50 uL), Red circular region:sender culture containing LasI gene(50 μL).Receiver culture containing LuxR-plux-gfp gene (50 μL)
-->more about Demo experiments detail
references
- M.K Winson et al.:Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing.FEMS Microbiology Letters 163 (1998) 185-192
- BBa_F2620:Specificity
- Paul Williams.:Quorum sensing, communication and cross-kingdom signalling in the bacterial world.Microbiology 153 (2007), 3923-3938
- Michiko E. Taga. Bonnie L.Bassler.:Chemical communication among bacteria.PNAS.November 25, 2003,100.suppl.2
 "
"