Team:ETH Zurich/Modeling/Overview
From 2008.igem.org
(→Overview on the modelling framework) |
(→Overview on the modelling framework) |
||
| Line 16: | Line 16: | ||
This page is meant to give an introduction to the the overall modelling framework we have constructed in order to asses feasibility analysis, temporal scale details and other parameter estimations that regard our project setup. As introduced in the [[Team:ETH_Zurich/Project/Overview|project overview section]], four main components can be identified in the devised mechanism. Accordingly, we divided the modelling framework in four modules that tack the relative problematics. | This page is meant to give an introduction to the the overall modelling framework we have constructed in order to asses feasibility analysis, temporal scale details and other parameter estimations that regard our project setup. As introduced in the [[Team:ETH_Zurich/Project/Overview|project overview section]], four main components can be identified in the devised mechanism. Accordingly, we divided the modelling framework in four modules that tack the relative problematics. | ||
<br> | <br> | ||
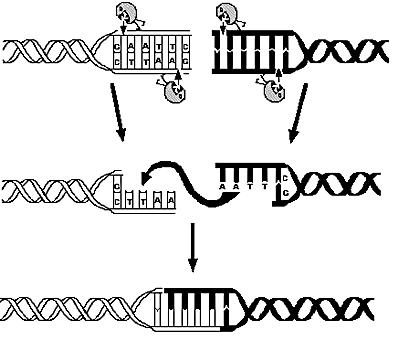
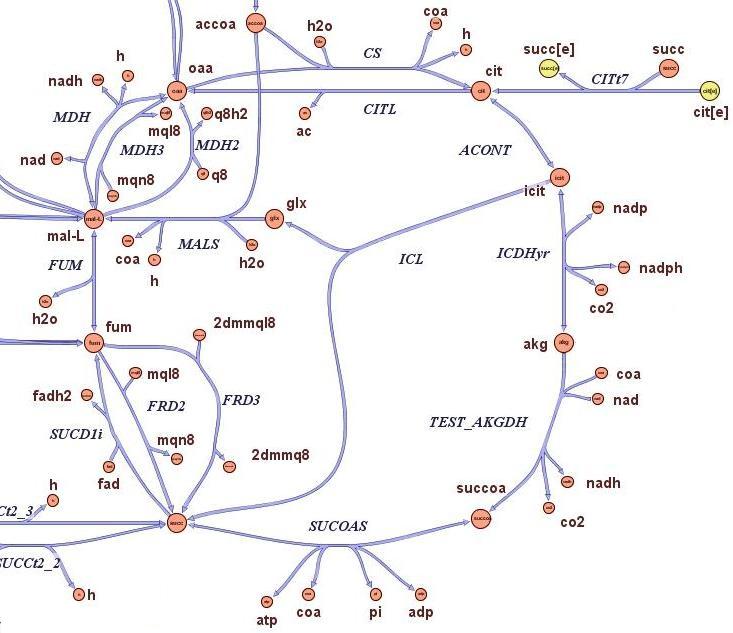
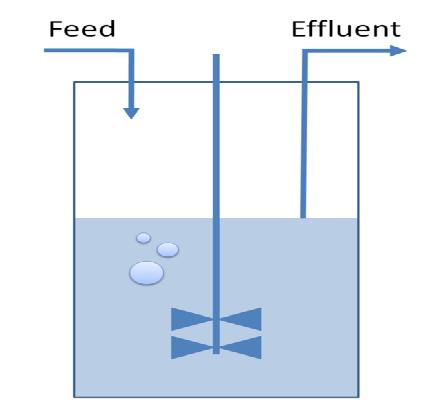
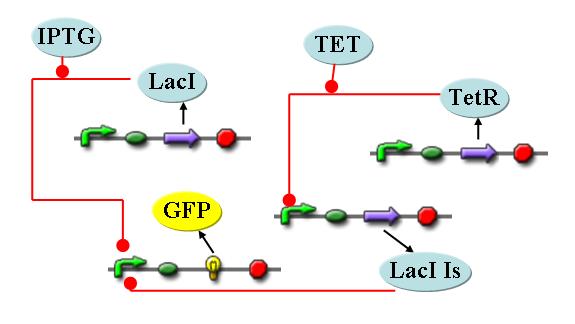
| - | The first module is concerned with the analysis of restriction enzymes and their cutting pattern on E.Coli genome, the second module | + | The first module is concerned with the analysis of restriction enzymes and their cutting pattern on E.Coli genome, the second module predicts the cell's response to the selection pressure and the forced genome reduction from a system point of view (that is, using a genome scale model), the third module addresses issues related to the sensitivity and setting of the chemostat mechanism, the fourth and final module presents the model of the genetic switch circuit used to control the restriction enzymes expression. |
<br> | <br> | ||
In the table below, you can find a bird-eye view on the four modules, with the most important aspects highlighted. Since we believe that a model is useful only when it answers specific and well-posed questions, this is the first aspect we report in the summary view. Second we briefly report about the modelling method applied. As last, we summarize the results we obtained. | In the table below, you can find a bird-eye view on the four modules, with the most important aspects highlighted. Since we believe that a model is useful only when it answers specific and well-posed questions, this is the first aspect we report in the summary view. Second we briefly report about the modelling method applied. As last, we summarize the results we obtained. | ||
Revision as of 10:07, 22 October 2008
Overview on the modelling frameworkThis page is meant to give an introduction to the the overall modelling framework we have constructed in order to asses feasibility analysis, temporal scale details and other parameter estimations that regard our project setup. As introduced in the project overview section, four main components can be identified in the devised mechanism. Accordingly, we divided the modelling framework in four modules that tack the relative problematics.
|
 "
"