Team:Harvard/Parts
From 2008.igem.org
(→mtrB) |
|||
| (96 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
| + | <html> | ||
| + | <head> | ||
| + | <style> | ||
| + | table { | ||
| + | background-color: #c4dbea; | ||
| + | font-color: #333333; | ||
| + | color:#333333; | ||
| + | } | ||
| + | a.menu { | ||
| + | background-color: #c4dbea; | ||
| + | color: #333333; | ||
| + | width: 12em; | ||
| + | } | ||
| + | |||
| + | .firstHeading { | ||
| + | color:#333333; | ||
| + | } | ||
| + | |||
| + | #bodyContent { | ||
| + | background-color: #c4dbea; | ||
| + | } | ||
| + | |||
| + | #content { | ||
| + | background-color: #c4dbea; | ||
| + | } | ||
| + | |||
| + | #footer-box { | ||
| + | background-color: #c4dbea; | ||
| + | } | ||
| + | p { | ||
| + | color:#333333; | ||
| + | } | ||
| + | body { | ||
| + | background-color:#333333; | ||
| + | } | ||
| + | .firstHeading { | ||
| + | display:none; | ||
| + | } | ||
| + | </style> | ||
| + | </head> | ||
| + | </html> | ||
| + | |||
| + | |||
{| | {| | ||
| align="center" style="background:#c4dbea"| | | align="center" style="background:#c4dbea"| | ||
| - | |||
<html><a href = "https://2008.igem.org/Team:Harvard"><img src="https://static.igem.org/mediawiki/2008/b/b9/Harvard_logo.png"></a></html> | <html><a href = "https://2008.igem.org/Team:Harvard"><img src="https://static.igem.org/mediawiki/2008/b/b9/Harvard_logo.png"></a></html> | ||
| - | {| style="color:#1b2c8a;background-color:#FFF;" cellpadding="0" cellspacing="0" border="0" bordercolor="#000" width="100%" align="center" | + | <br> |
| - | + | ||
| - | + | {{Template:Main}} | |
| - | + | ||
| - | + | {| style="color:#1b2c8a;background-color:#FFF;" cellpadding="0" cellspacing="0" border="0" bordercolor="#000" width="100%" align="center"|} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |} | + | |
<!--- body here---> | <!--- body here---> | ||
| - | {|align="justify" style="background-color:#FFFFFF;text-indent: 15pt;text-align:justify" cellpadding="50" width=" | + | {|align="justify" style="background-color:#FFFFFF;text-indent: 15pt;text-align:justify" cellpadding="50" width="90%" |
| | | | ||
| - | |||
| - | + | =Parts Submitted to Registry= | |
| + | You can find the complete list of parts we submitted to the registry [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2008&group=Harvard here]. | ||
| + | ==''mtrB''== | ||
| + | Many genes are involved in ''S. oneidensis''’s complex respiratory system (Heidelberg et al. 2002). We focused on ''mtrB'', which encodes a 679-amino-acid-long outer membrane protein involved in the binding of metals and the localization of outer membrane cytochromes during reduction (Bretschger et al. 2007). It is unfortunately toxic in ''E. coli'' (Saffarini). Bretschger et al. recently characterized the role of mtrB in anaerobic respiration of ''S. oneidensis'' by looking at the effects of knock-out and complementation of mtrB on the electrical output of ''S. oneidensis''. It was found that the strain which lacked mtrB produced less than 20% of the current generated by the wild type strain. In complemented strains, where mtrB is expressed constitutively under the control of the lacZ promoter in the knock-out strain, the phenotype was rescued with a similar amount of current being produced to that of the wild type (Bretschger et al. 2007). Not only does this experiment demonstrate the importance of mtrB in reduction in ''S. oneidensis'', it also suggests a mechanism by which this electrical output could be controlled. Transforming plasmids containing mtrB under the control of an inducible promoter into mtrB knock out ''S. oneidensis'', would conceivably create a strain of ''S. oneidensis'' which could increase its electrical output in response to the turning-on of the promoter controlling mtrB expression. The creation of a strain with an inducible electrical output could have important applications in biotechnology by creating a system which couples the ability of ''S. oneidensis'' to respond to a diverse array of stimuli with the speed and ubiquity of electricity. | ||
| + | |||
| + | Since we found construction intermediates from the registry to be especially useful, we provided our intermediates with mtrB with RBS, terminator, or both. | ||
| + | |||
| + | ==The Genetic Circuitry== | ||
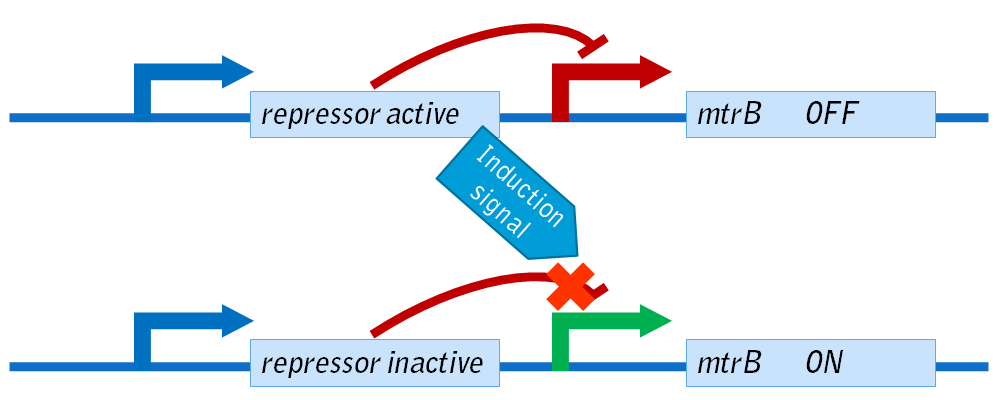
| + | In order to control the expression of exogenous mtrB we sought to create several different inducible systems. As depicted below, these systems consist of a repressor under the control of a constitutive promoter (blue). In the default state, the repressor will bind to the downstream promoter (red), preventing RNA polymerase from attaching to the DNA strand to start transcription. Thus, in this state, mtrB is not expressed. | ||
| + | |||
| + | In the presence of an inducer, mtrB expression should occur. In this case, the inducer binds the repressor protein, preventing it from binding to the operator within the promoter. RNA polymerase is therefore able to bind to the promoter (green), allowing for expression of mtrB. | ||
| + | |||
| + | <div style="text-indent:0pt">[[Image:Harvsystem.png|thumb|650px|center|Induction results in mtrB expression]] | ||
| + | </div> | ||
| + | |||
| - | + | We tried to create such systems capable of being induced by [[Team:Harvard/Parts/LacI| IPTG]], | |
| - | | | + | [[Team:Harvard/Parts/Tempsenseci| heat]], [[Team:Harvard/Parts/Other| tetracycline, and light]]. |
| - | + | ||
| - | + | ||
| - | | | + | |
Latest revision as of 03:58, 30 October 2008
|
|
 "
"