Parts Submitted to Registry
Short intro
See [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2008&group=Harvard list] of all parts we submitted.
\\******change subsection headers*******\\
General overview of mtrB
Many genes are involved in Shewanella’s complex respiratory system (Heidelberg et al. 2002). We focused on mtrB, a 679-amino-acid-long outer membrane protein thought to be involved in the binding of metals and the localization of outer membrane cytochromes during reduction (Bretschger et al. 2007). It is unfortunately toxic in E. coli (Saffarini). Bretschger et al. recently characterized the role of mtrB in anaerobic respiration of Shewanella by looking at the effects of knock-out and complementation of mtrB on the electrical output of Shewanella. It was found that the strain which lacked mtrB produced less than 20% of the current generated by the wild type strain. In complemented strains, where mtrB is expressed constitutively under the control of the lacZ promoter in the knock-out strain, the phenotype was rescued with a similar amount of current being produced to that of the wild type (Bretschger et al. 2007). Not only does this experiment demonstrate the importance of mtrB in reduction in Shewanella, it also suggests a mechanism by which this electrical output could be controlled. Transforming plasmids containing mtrB under the control of an inducible promoter into mtrB knock out Shewanella, would conceivably create a strain of Shewanella which could increase its electrical output in response to the turning-on of the promoter controlling mtrB expression. The creation of a strain with an inducible electrical output could have important applications in biotechnology by creating a system which couples the ability of Shewanella to respond to a diverse array of stimuli with the speed and ubiquity of electricity.
General overview of QPIs
Lac system (will be moved to separate page)
In this system, the lac repressor (LacI) is controlled by a strong constitutive promoter, and is upstream of mtrB under the control of pLac, a LacI regulated promoter. In the default state, LacI is expressed, and inhibits transcription at pLac. Thus, in the default state, mtrB is not expressed. IPTG (an analog of allolactose) induces mtrB expression by binding to LacI, thereby preventing it from inhibiting transcription at pLac.
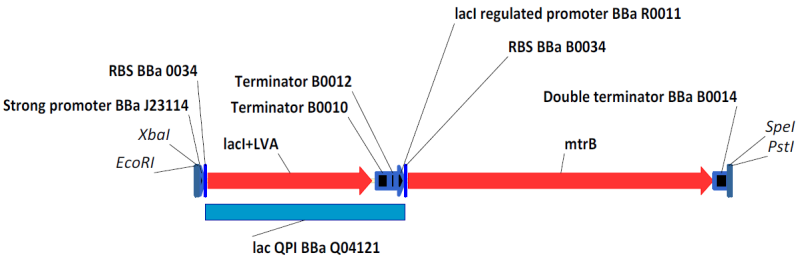 BBa_K098984 with BioBrick Prefix and Suffix Inducibility Test for Lac System with GFP Reporter and Weak Promoter
An induction test was designed to test the inducibility of the lac system by IPTG when the repressor (LacI) is driven by a weak promoter.
Experimental Design
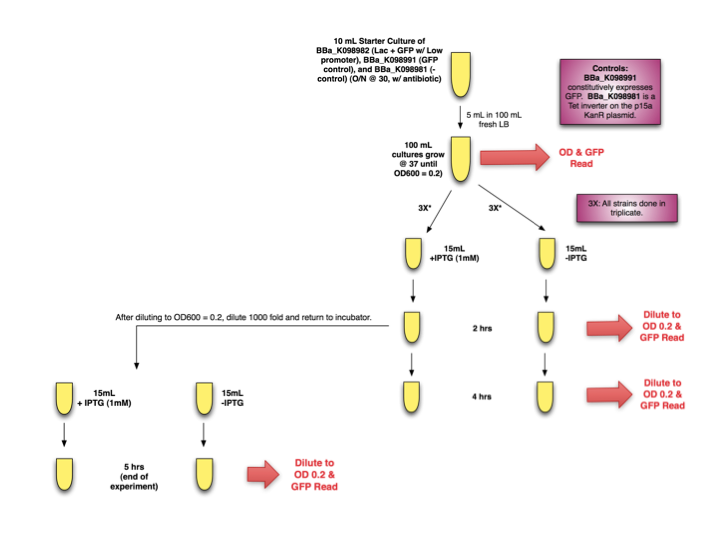 Lac Induction Experimental Design
Starter cultures of E. coli with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K098982| BBa_K098982] were grown overnight. They were then diluted and grown to OD 0.2 before separation into induced (+IPTG, 1mM) and uninduced cultures (-IPTG). OD and GFP readings were taken at time 0, 2, and 4 hours. Additionally, after diluting T=2hrs samples to OD 0.2 for accurate GFP measurements, samples were further diluted 1000x, induced (or not induced) again, and placed back in the incubator until the end of the experiment, when OD and GFP readings were taken.
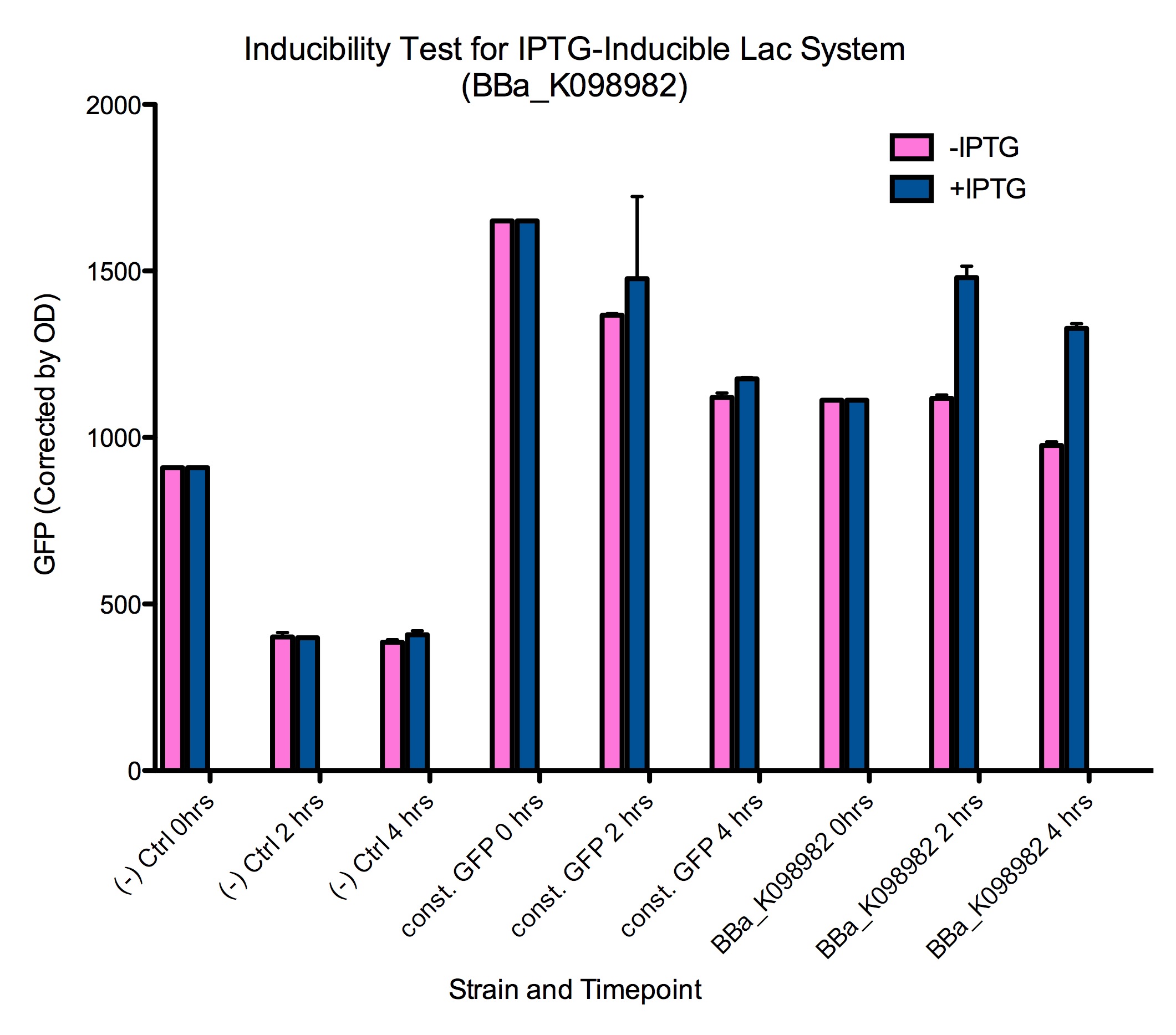
Results
Induction of GFP expression was observed at both 2 and 4 hours after adding IPTG. Levels of GFP expression in uninduced samples, however, remained relatively the same throughout the 4 hours. Meanwhile, IPTG induction was not observed in either the negative control ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K098981| BBa_K098981]) or the constitutive GFP control ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K098991| BBa_K098991]).
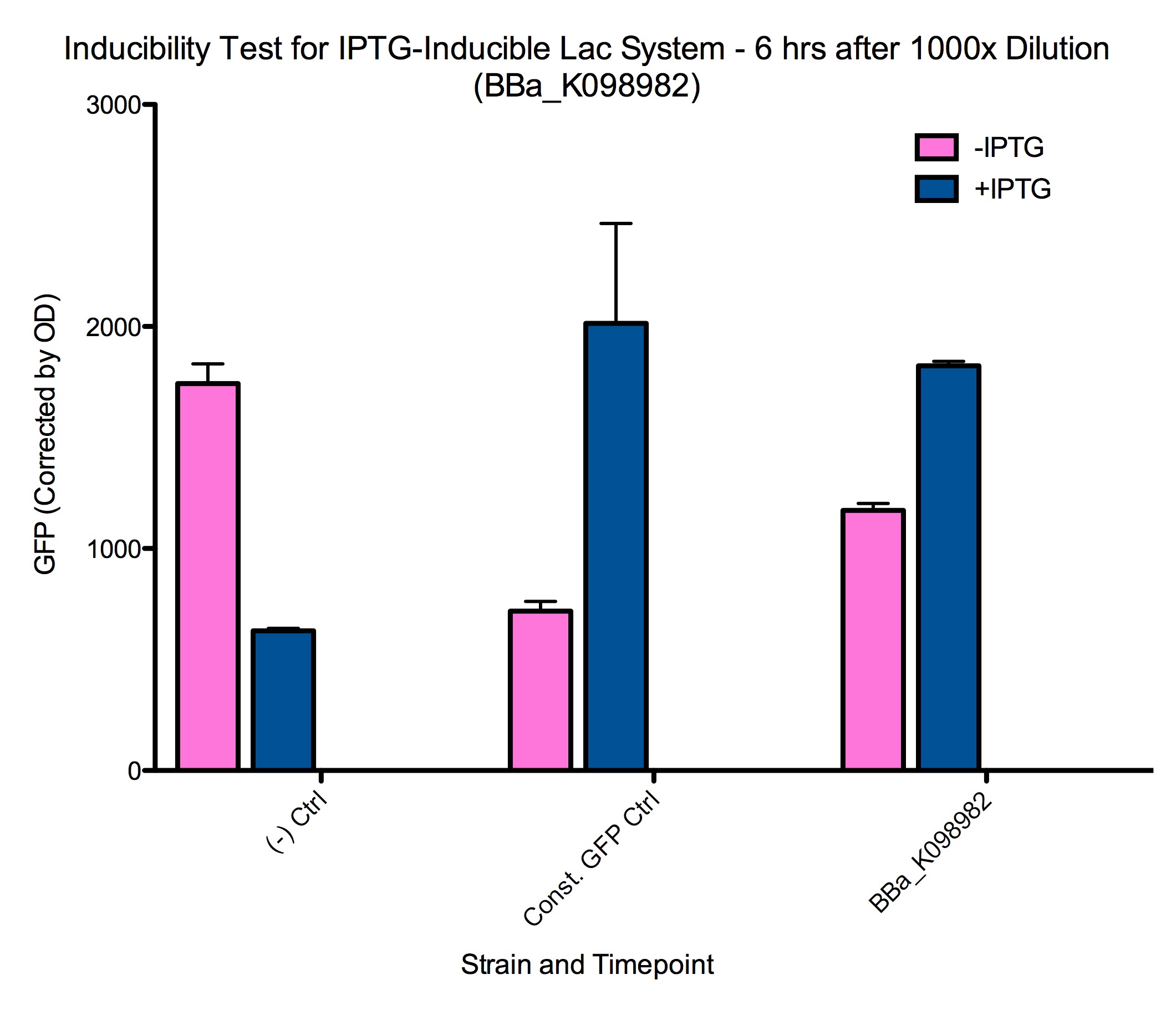
The 1000x dilutions of samples at 2 hours after induction produced similar results after 5-6 hours. These cells are presumably at a different growth phase from cells that have been growing at much higher concentration for longer, and are thus induced in different conditions.
QPIs that we did not use with mtrB
tet
Thermoinducible cI System
This system uses a a temperature sensitive variant of cI lambda to regulate the lambda promoter.
The thermoinducible cI lambda system uses cI857 (a mutant form of cI from [http://www.addgene.org/pgvec1?f=c&vectorid=5079&cmd=genvecmap&dim=800&format=html&mtime=1188314819| pGW7] purchased from [http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx#40554| ATCC]) to regulate expression of genes under the control of the lambda promoter. The cI857 repressor is repressed by thermal denaturation. Activity of cI857 begins to decrease around 30 ºC and is fully denatured by around 42 ºC (Leipold et al., 1994). Thus transcription of the gene under the control of the lambda promoter can be induced by increasing the temperature from 30 ºC to 37 ºC-40 ºC.
BBa_K098995
This is a thermosensitive cI inducible system driven by a strong promoter.
BBa_K098993
This is a thermosensitive cI inducible system driven by a weak promoter.
Inducibility Test for Thermosensitive cI Systems with GFP Reporters
An induction test was designed to test the inducibility of the heat sensitive cI systems. Two constructs were made:
BBa_K098988
This is a thermosensitive cI inducible system driven by a strong promoter and with a GFP indicator.
BBa_K098988
This is a thermosensitive cI inducible system driven by a weak promoter and with a GFP indicator.
Experimental Design
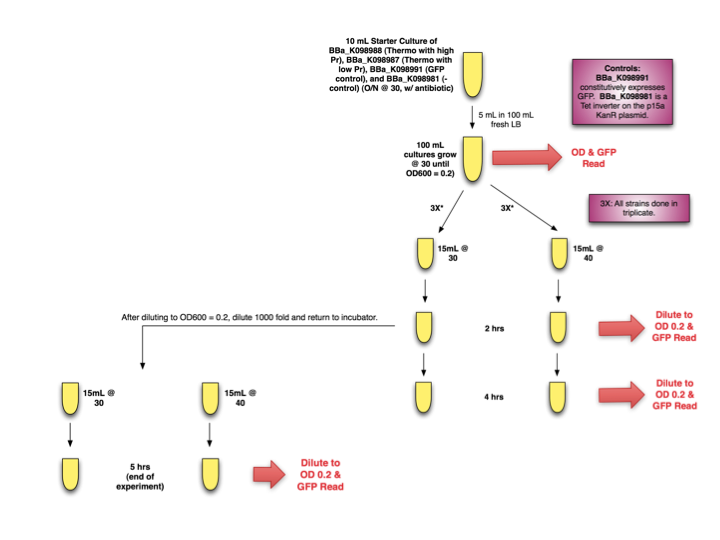 Thermo Induction Experimental Design
Starter cultures of E. coli with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K098988| BBa_K098988] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K098987| BBa_K098987] were grown overnight. They were then diluted and grown to OD 0.2 before separation into induced (40 ºC) and uninduced cultures (30 ºC). OD and GFP readings were taken at time 0, 2, and 4 hours. Additionally, after diluting T=2hrs samples to OD 0.2 for accurate GFP measurements, samples were further diluted 1000x, induced (or not induced) again, and placed back in their respective incubators until the end of the experiment, when OD and GFP readings were taken.
Results
Slight induction of GFP expression was observed at both 2 and 4 hours after moving samples to 40 ºC. However, since levels of GFP expression in the GFP control ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K098991| BBa_K098991]) went down in samples at 40 ºC, it is possible that the heat affects the ability of constitutive GFP reporters to generate GFP expression. This makes it unclear how much GFP expression is actually induced by the temperature change. A slight decrease in baseline fluorescence was also observed in negative control cells ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K098981| BBa_K098981]).
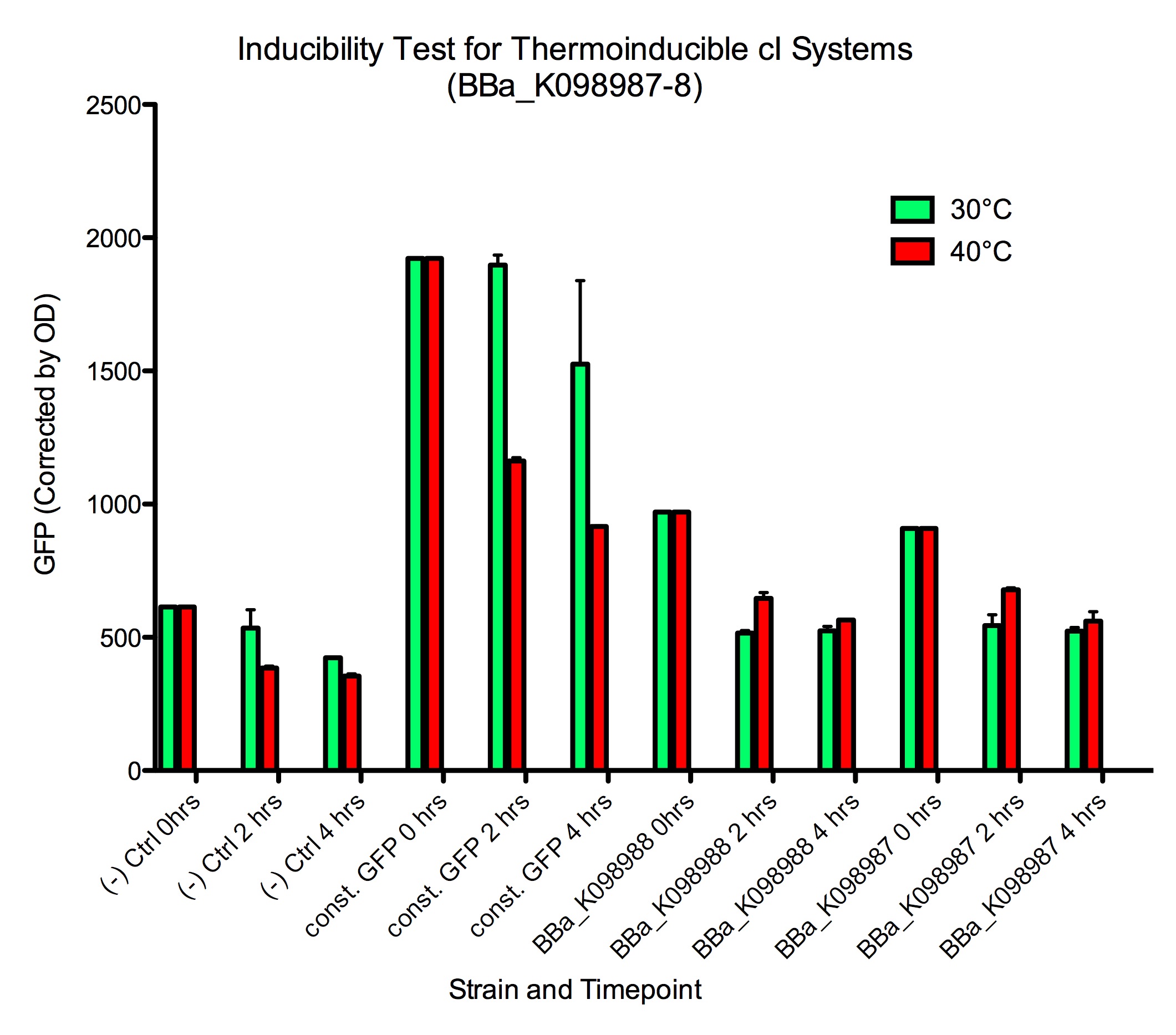
Results from the 1000x dilutions were inconclusive because the E. coli grows much slower at 30 ºC than at 40 ºC, so by the end of the experiment, there was a vast difference in cell concentration between the two sets of samples.
Edit stuff here; we'll move entire section to new page.
|