Team:Heidelberg/Notebook/Killing I/Notebook/week11
From 2008.igem.org
(New page: <html> <link rel='stylesheet' href='http://igem-heidelberg.de/fileadmin/Wiki/Heidelberg2.css' type='text/css' /> <link rel="stylesheet" href="http://igem-heidelberg.de/fileadmin/Wiki/Menu....) |
(→Characterization of oriT) |
||
| (12 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
<html> | <html> | ||
| - | <link | + | |
| - | + | ||
| - | + | <style> | |
| + | h1.firstHeading { display: none; } | ||
| + | |||
| + | p {text-align: justify;} | ||
| + | |||
| + | a:link { color: #00b0e6; text-decoration: none} | ||
| + | a:visited { color:#00b0e6; text-decoration: none} | ||
| + | a:hover { color:#f29400; text-decoration: none} | ||
| + | a:active { color:#f29400; text-decoration: none} | ||
| + | |||
| + | #bodyContent { padding: 10px auto; width: 910px; margin: auto; clear: none; } | ||
| + | |||
| + | table#team_members { text-align: justify; border: 0; } | ||
| + | table#team_members h2, table#team_members h3 { clear: both; } | ||
| + | |||
| + | |||
| + | /*-----------------------------------------------------------------------------------------------*/ | ||
| + | div.MenuBar ul li ul.DropDownMenu { | ||
| + | display: none; /* Hides all drop-down menus. */ | ||
| + | |||
| + | } | ||
| + | /* | ||
| + | li:hover works in IE7 and FF2. | ||
| + | a:hover works in IE6 and FF2. | ||
| + | a:hover breaks li:hover in FF2. | ||
| + | */ | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li ul.SideMenu, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a ul.SideMenu { | ||
| + | display: none; /* Hides all side menus. */ | ||
| + | } | ||
| + | /*------------------------------------------------------------------------------------- Menu Bar */ | ||
| + | div.MenuBar { | ||
| + | font: arial, helvetica, sans-serif; | ||
| + | height: 30px; | ||
| + | width: 910px; | ||
| + | /*width: 100%*/ | ||
| + | margin: 0; | ||
| + | border-top: 0; | ||
| + | border-right: 0; | ||
| + | border-left: 0; | ||
| + | padding: 0; | ||
| + | background: black; | ||
| + | |||
| + | } | ||
| + | div.MenuBar ul { | ||
| + | font: arial, helvetica, sans-serif; | ||
| + | text-align: center; | ||
| + | list-style-type: none; | ||
| + | margin: 0.5em auto; | ||
| + | border: 0; | ||
| + | padding: 0; | ||
| + | background: black; | ||
| + | } | ||
| + | div.MenuBar ul li { | ||
| + | font: arial, helvetica, sans-serif; | ||
| + | display: block; | ||
| + | padding: 0; | ||
| + | margin: 0; | ||
| + | font-size: 1.3em; | ||
| + | float: left; | ||
| + | background: black; | ||
| + | text-align: center; | ||
| + | width: 107px; | ||
| + | position: relative; /* Sets the positioning context for each drop-down menu. */ | ||
| + | } | ||
| + | |||
| + | div.MenuBar ul li a { | ||
| + | font: arial, helvetica, sans-serif; | ||
| + | display: block; | ||
| + | background: black; | ||
| + | height: 22px; /* Keep height + padding-top + padding-bottom sync with the menu bar height. */ | ||
| + | color: #ffffff; | ||
| + | padding-top: 4px; | ||
| + | padding-bottom: 4px; | ||
| + | padding-left: 1em; /* Sets the left space between top-level items. */ | ||
| + | padding-right: 1em; /* Sets the right space between top-level items. */ | ||
| + | text-decoration: none; | ||
| + | } | ||
| + | |||
| + | /*------------------------------------------------------------------------------ Drop-Down Menus */ | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu { | ||
| + | display: block; | ||
| + | width: 10em; /* Drop-down menu width. | ||
| + | Use MenuTailor.css to customize. */ | ||
| + | height: 1em; | ||
| + | padding: 1px; /* Sets the drop-down menu "effective border" width. */ | ||
| + | position: absolute; | ||
| + | top: 23px; /* Places the drop-down menu under the menu bar. | ||
| + | Keep it sync with the menu bar height. */ | ||
| + | left: 0; /* Aligns the drop-down menu to its top-level item. */ | ||
| + | background-color: black; /* Selected item. */ | ||
| + | color: #FFFFFF; | ||
| + | |||
| + | } | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a { | ||
| + | width: 10em; /* Keep it sync with the drop-down menu width. | ||
| + | Use MenuTailor.css to customize. */ | ||
| + | height: 1em; | ||
| + | padding-left: 0; | ||
| + | padding-right: 0; | ||
| + | background-color: black; /* Selected item. */ | ||
| + | color: #FFFFFF; | ||
| + | } | ||
| + | ul.DropDownMenu li a span { | ||
| + | display: block; | ||
| + | padding-left: 0.75em; /* Sets the left space of each drop-down menu item. */ | ||
| + | padding-right: 0.25em; /* Sets the right space of each drop-down menu item. */ | ||
| + | text-align: right; /* Aligns the >> symbol to the right. */ | ||
| + | } | ||
| + | ul.DropDownMenu li a span span { | ||
| + | float: left; /* Aligns the text (back) to the left. */ | ||
| + | font: 12px arial, helvetica, sans-serif; /* Required for IE55. */ | ||
| + | height: 20px; | ||
| + | color: #FFFFFF; | ||
| + | } | ||
| + | /*----------------------------------------------------------------------------------- Side Menus */ | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu { | ||
| + | display: block; | ||
| + | width: 11em; /* Side menu width. | ||
| + | Use MenuTailor.css to customize. */ | ||
| + | padding: 1px; /* Sets the side menu "effective border" width. */ | ||
| + | position: absolute; | ||
| + | top: -1px; /* Aligns the side menu to its drop-down menu item. | ||
| + | Keep it sync with the side menu "effective border" width. */ | ||
| + | left: 13em; /* Places the side menu to the right of the drop-down menu. | ||
| + | Keep it sync with the drop-down menu width. | ||
| + | Use MenuTailor.css to customize. */ | ||
| + | } | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu li a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu li a { | ||
| + | width: 11em; /* Keep it sync with the side menu width. | ||
| + | Use MenuTailor.css to customize. */ | ||
| + | font: 12px arial, helvetica, sans-serif; /* Required for IE55. */ | ||
| + | left: 13em; /* Places the side menu to the right of the drop-down menu. | ||
| + | Keep it sync with the drop-down menu width. | ||
| + | Use MenuTailor.css to customize. */ | ||
| + | } | ||
| + | div.MenuBar ul li ul.DropDownMenu li ul.SideMenu li a span { | ||
| + | padding-left: 1.5em; /* Sets the left space of each side menu item. */ | ||
| + | padding-right: 0.5em; /* Sets the right space of each side menu item. */ | ||
| + | text-align: left; | ||
| + | font: 12px arial, helvetica, sans-serif; /* Required for IE55. */ | ||
| + | left: 13em; /* Places the side menu to the right of the drop-down menu. | ||
| + | Keep it sync with the drop-down menu width. | ||
| + | Use MenuTailor.css to customize. */ | ||
| + | } | ||
| + | /*----------------------------------------------------------------------------- Browser Specific */ | ||
| + | * html div.MenuBar ul li a { | ||
| + | float: left; /* Required for IE55 and IE6. | ||
| + | Breaks O9. | ||
| + | Hidden (* html) from non-IE browsers. */ | ||
| + | } | ||
| + | * html ul.DropDownMenu li a:hover { | ||
| + | cursor: hand; /* Required for IE55. | ||
| + | Hidden (* html) from non-IE browsers. */ | ||
| + | } | ||
| + | ul.DropDownMenu li a:hover { | ||
| + | cursor: pointer; /* Required for IE6 and IE7. | ||
| + | Hidding it (* html) from non-IE browsers breaks IE7! | ||
| + | } | ||
| + | * html div.MenuBar a:hover { | ||
| + | text-decoration: none; /* Required for IE55 and IE6. | ||
| + | Hidden (* html) from non-IE browsers. */ | ||
| + | } | ||
| + | * html div.MenuBar ul li table, | ||
| + | * html div.MenuBar ul li table td { | ||
| + | border: 0; /* Required for IE55 and IE6. | ||
| + | Hidden (* html) from non-IE browsers. */ | ||
| + | } | ||
| + | /*------------------------------------------------------------------------------- Default Colors */ | ||
| + | div.MenuBar { | ||
| + | background-color: Menu; | ||
| + | border-bottom: 1px solid ButtonShadow; | ||
| + | } | ||
| + | div.MenuBar a { | ||
| + | background-color: Menu; /* Top-level unselected items. */ | ||
| + | color: MenuText; | ||
| + | } | ||
| + | div.MenuBar ul li:hover a, | ||
| + | div.MenuBar ul li a:hover { | ||
| + | color: #ea7f16; | ||
| + | background-color: Highlight; /* Top-level selected item. */ | ||
| + | color: HighlightText; | ||
| + | } | ||
| + | /*...............................................................................................*/ | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu { | ||
| + | background-color: ButtonShadow; /* Sets the drop-down menu "effective border" color. */ | ||
| + | } | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a { | ||
| + | background-color: Menu; Drop-down menu unselected items. | ||
| + | Sets the drop-down menu "effective background" color. */ | ||
| + | color: MenuText; | ||
| + | } | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover { | ||
| + | background-color: Highlight; /* Drop-down menu selected item. */ | ||
| + | color: HighlightText; | ||
| + | } | ||
| + | /*...............................................................................................*/ | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu { | ||
| + | background-color: ButtonShadow; /* Sets the side menu "effective border" color. */ | ||
| + | } | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu li a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu li a { | ||
| + | background-color: Menu; /* Side menu unselected items. | ||
| + | Sets the side menu "effective background" color. */ | ||
| + | color: MenuText; | ||
| + | } | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu li a:hover, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu li a:hover { | ||
| + | background-color: Highlight; /* Side menu selected item. */ | ||
| + | color: HighlightText; | ||
| + | } | ||
| + | /*-----------------------------------------------------------------------------------------------*/ | ||
| + | /*Script-Free 3-Level Menu 1.2 Tailor | ||
| + | www.CesarDaniel.info | ||
| + | /*-------------------------------------------------------------------------------------- General */ | ||
| + | body { | ||
| + | background: white; | ||
| + | color: black; | ||
| + | margin: 0; | ||
| + | border: 0; | ||
| + | padding: 0; | ||
| + | } | ||
| + | |||
| + | |||
| + | div.MenuBar { | ||
| + | font: 13px arial, helvetica, sans-serif; | ||
| + | } | ||
| + | div.MenuBar ul { | ||
| + | font: 13px arial, helvetica, sans-serif; /* Required for IE55. */ | ||
| + | } | ||
| + | /*--------------------------------------------------------------------------------------- Colors */ | ||
| + | div.MenuBar { | ||
| + | background-color: black; /* Selected item. */ | ||
| + | color: #FFFFFF; | ||
| + | border-bottom: 1px solid ButtonShadow; | ||
| + | } | ||
| + | div.MenuBar a, | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a, | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu li a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu li a { | ||
| + | background-color: black; /* Selected item. */ | ||
| + | color: #FFFFFF; | ||
| + | } | ||
| + | div.MenuBar ul li:hover a, | ||
| + | div.MenuBar ul li a:hover, | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover a, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover, | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu li a:hover, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu li a:hover { | ||
| + | background-color: #00b0e6; /* Selected item. */ | ||
| + | color: #FFFFFF; | ||
| + | } | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu, | ||
| + | div.MenuBar ul li:hover ul.DropDownMenu li:hover ul.SideMenu, | ||
| + | div.MenuBar ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu { | ||
| + | background-color: ButtonShadow; /* Sets the drop-down and side menus "effective border" color. */ | ||
| + | } | ||
| + | /*--------------------------------------------------------------------------------------- Widths */ | ||
| + | /* | ||
| + | |||
| + | /* | ||
| + | Menu Bar 1 | ||
| + | Drop-Down Menu #2 | ||
| + | */ | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu#MB1-DDM4, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu#MB1-DDM4, | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu#MB1-DDM4 li a, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu#MB1-DDM4 li a { | ||
| + | width: 11em; /* Drop-down menu width. */ | ||
| + | } | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu#MB1-DDM5, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu#MB1-DDM5, | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu#MB1-DDM5 li a, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu#MB1-DDM5 li a { | ||
| + | width: 12em; /* Drop-down menu width. */ | ||
| + | } | ||
| + | |||
| + | /*...............................................................................................*/ | ||
| + | /* | ||
| + | Menu Bar 1 | ||
| + | Drop-Down Menu #2 | ||
| + | Side Menu #1 | ||
| + | */ | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM1, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM1 { | ||
| + | left: 15.5em !important; /* Places the side menu to the right of the drop-down menu. | ||
| + | Keep it sync with the drop-down menu width. */ | ||
| + | } | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM1, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM1, | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM1 li a, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM1 li a { | ||
| + | width: 10em; /* Side menu width. */ | ||
| + | } | ||
| + | /*...............................................................................................*/ | ||
| + | /* | ||
| + | Menu Bar 1 | ||
| + | Drop-Down Menu #2 | ||
| + | Side Menu #2 | ||
| + | */ | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM2, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM2 { | ||
| + | left: 15.5em !important; /* Places the side menu to the right of the drop-down menu. | ||
| + | Keep it sync with the drop-down menu width. */ | ||
| + | } | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM2, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM2, | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM2 li a, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM2 li a { | ||
| + | width: 10em; /* Side menu width. */ | ||
| + | } | ||
| + | /*...............................................................................................*/ | ||
| + | /* | ||
| + | Menu Bar 1 | ||
| + | Drop-Down Menu #2 | ||
| + | Side Menu #3 | ||
| + | */ | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM3, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM3 { | ||
| + | left: 15.5em !important; /* Places the side menu to the right of the drop-down menu. | ||
| + | Keep it sync with the drop-down menu width. */ | ||
| + | } | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM3, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM3, | ||
| + | div.MenuBar#navi ul li:hover ul.DropDownMenu li:hover ul.SideMenu#MB1-DDM2-SM3 li a, | ||
| + | div.MenuBar#navi ul li a:hover ul.DropDownMenu li a:hover ul.SideMenu#MB1-DDM2-SM3 li a { | ||
| + | width: 10em; /* Side menu width. */ | ||
| + | } | ||
| + | /*...............................................................................................*/ | ||
| + | |||
| + | </style> | ||
| + | |||
<body> | <body> | ||
| Line 52: | Line 393: | ||
</li> | </li> | ||
<li> | <li> | ||
| - | <a href="https://2008.igem.org/Team:Heidelberg/ | + | <a href="https://2008.igem.org/Team:Heidelberg/Modeling" style="color: white">Modeling<!--[if gt IE 6]><!--></a><!--<![endif]--> |
<!--[if lt IE 7]><table border="0" cellpadding="0" cellspacing="0"><tr><td><![endif]--> | <!--[if lt IE 7]><table border="0" cellpadding="0" cellspacing="0"><tr><td><![endif]--> | ||
<ul class="DropDownMenu" id="MB1-DDM3"> | <ul class="DropDownMenu" id="MB1-DDM3"> | ||
| Line 62: | Line 403: | ||
</li> | </li> | ||
<li> | <li> | ||
| - | <a href="https://2008.igem.org/Team:Heidelberg/Notebook/ | + | <a href="https://2008.igem.org/Team:Heidelberg/Notebook/Overview" style="color: white">Notebook<!--[if gt IE 6]><!--></a><!--<![endif]--> |
<!--[if lt IE 7]><table border="0" cellpadding="0" cellspacing="0"><tr><td><![endif]--> | <!--[if lt IE 7]><table border="0" cellpadding="0" cellspacing="0"><tr><td><![endif]--> | ||
<ul class="DropDownMenu" id="MB1-DDM5"> | <ul class="DropDownMenu" id="MB1-DDM5"> | ||
| Line 98: | Line 439: | ||
</li> | </li> | ||
<li style="width: 160px"> | <li style="width: 160px"> | ||
| - | <a href="https://2008.igem.org/Team:Heidelberg/ | + | <a href="https://2008.igem.org/Team:Heidelberg/Human_Practice/Project_Overview" style="color: white">Human Practice<!--[if gt IE 6]><!--></a><!--<![endif]--> |
<!--[if lt IE 7]><table border="0" cellpadding="0" cellspacing="0"><tr><td><![endif]--> | <!--[if lt IE 7]><table border="0" cellpadding="0" cellspacing="0"><tr><td><![endif]--> | ||
<ul class="DropDownMenu" id="MB1-DDM4"> | <ul class="DropDownMenu" id="MB1-DDM4"> | ||
| Line 106: | Line 447: | ||
<li><a href="https://2008.igem.org/Team:Heidelberg/Human_Practice/Surveys"><span><span>Surveys</span></span></a></li> | <li><a href="https://2008.igem.org/Team:Heidelberg/Human_Practice/Surveys"><span><span>Surveys</span></span></a></li> | ||
<li><a href="https://2008.igem.org/Team:Heidelberg/Human_Practice/Open_Day"><span><span>Open Day</span></span></a></li> | <li><a href="https://2008.igem.org/Team:Heidelberg/Human_Practice/Open_Day"><span><span>Open Day</span></span></a></li> | ||
| - | <li><a href="https://2008.igem.org/Team:Heidelberg/Human_Practice/Nobel_Prize"><span><span>Nobel Prize</span></span></a></li> | + | <li><a href="https://2008.igem.org/Team:Heidelberg/Human_Practice/Nobel_Prize"><span><span>Nobel Prize</span></span></a></li> |
| + | |||
</ul> | </ul> | ||
<!--[if lte IE 6]></td></tr></table></a><![endif]--> | <!--[if lte IE 6]></td></tr></table></a><![endif]--> | ||
| Line 120: | Line 462: | ||
</body> | </body> | ||
</html> | </html> | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- bgcolor=white | ||
| + | ! height=20px, width=250px | [[Team:Heidelberg/Notebook/Killing_I/Notebook/week10|<< Week 10]]|| width=500px | [[Team:Heidelberg/Notebook/Killing_I/Notebook|Overview]]|| width=250px | [[Team:Heidelberg/Notebook/Killing_I/Notebook/week12| Week 12 >> ]] | ||
| + | |-style="height:20px" | ||
| + | |} | ||
'''Week 11''' | '''Week 11''' | ||
| - | |||
| - | |||
| - | |||
==Monday, 10/13/08 == | ==Monday, 10/13/08 == | ||
| - | ===Proceeding of | + | ===Proceeding of phage cloning strategy two=== |
*Digestion of the miniprep 1-6,9,10 from sunday and the original pBluescript with insert | *Digestion of the miniprep 1-6,9,10 from sunday and the original pBluescript with insert | ||
| Line 238: | Line 583: | ||
| - | === | + | ===Phage cloning strategy two=== |
*Mutagenesis PCR of pBlue with insert to remove KpnI restriction site | *Mutagenesis PCR of pBlue with insert to remove KpnI restriction site | ||
**using turbo Pfu | **using turbo Pfu | ||
| Line 246: | Line 591: | ||
| - | === | + | ===Phage cloning strategy one=== |
* mutation of old insert in pBluescript | * mutation of old insert in pBluescript | ||
* pBluescript + insert was cut with BamHI --> the resulting backbone cut out of the gel and religated. With this vector 2 mutagenesis PCRs were done to eleminate the two remaining XbaI restriction sites. | * pBluescript + insert was cut with BamHI --> the resulting backbone cut out of the gel and religated. With this vector 2 mutagenesis PCRs were done to eleminate the two remaining XbaI restriction sites. | ||
| Line 252: | Line 597: | ||
[[Image:Hd-phage-08-10-17_pBlue_insert_fully_mutated_XbaI-XhoI.jpg]] | [[Image:Hd-phage-08-10-17_pBlue_insert_fully_mutated_XbaI-XhoI.jpg]] | ||
| - | + | ===Characterization of oriT=== | |
| + | *Inoculate the cells for conjugation test<br> | ||
| + | 5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33<br> | ||
| + | 5ml LB/kanamycin/ampicilin +glycerol stock Top10 J01103+pUB307 | ||
==Wednesday, 10/15/08== | ==Wednesday, 10/15/08== | ||
| - | === | + | ===Phage cloning strategy two=== |
*inoculation of KpnI mutagenesis PCR samples --> Miniprep | *inoculation of KpnI mutagenesis PCR samples --> Miniprep | ||
*Digestion with KpnI/AgeI | *Digestion with KpnI/AgeI | ||
| Line 261: | Line 609: | ||
*Gel purification kit | *Gel purification kit | ||
| - | + | ===Characterization of oriT=== | |
| + | *Quantitatively test for oriT<br> | ||
| + | Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 2.08<br> | ||
| + | Recipient: overnight culture Top10 pBAD 33 OD(600nm): 2.24<br> | ||
| + | **Centrifuge 350ul overnight culture in 1.5ml eppi for 2min at 13000rpm, 8samples donor, 8samples recipient | ||
| + | **Wash the pellet twice with LB medium | ||
| + | **Resolve the pellet in 350ul LB medium | ||
| + | **Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid | ||
| + | **Add the washed donor suspension | ||
| + | **Vortex and resolve the pellet | ||
| + | **Centrifuge the mix for 1min at 13000rpm | ||
| + | **Resolve the pellet in 100ul LB | ||
| + | **Put membrane filter on the LB agar | ||
| + | **Pipett the suspension on membrane filter (8samples) | ||
| + | **Incubate the plates with membrane filter at 37°C | ||
| + | **Put directly one membrane filter into 1ml LB in an 1.5ml eppi | ||
| + | **Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min) | ||
| + | **After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps. | ||
| + | **Negative control plates: | ||
| + | ***LB/Cm+Amp: | ||
| + | <br>100ul donor overnight culture<br> | ||
| + | 100ul recipient overnight culture<br> | ||
| + | **Cell number determination | ||
| + | ***LB/Cm: 100ul 10-6 recipient overnight culture | ||
| + | ***LB/Kan+Amp: 100ul 10-6 donor overnight culture | ||
| + | <br> | ||
| + | *Result: | ||
| + | **Negative control: negative | ||
| + | **Colony on LB/Cm: 238 (Titer of recipient: 2.38e9/ml) | ||
| + | **Colony on LB/Kan+Amp: 99 (Titer of donor: 0.99e9/ml) | ||
| + | **Colony on other LB/Cm+Amp plates: | ||
| + | ***10-5 dilute: <br> | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=20px. width=200px | Time || width=250px | Colony | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |0 || 9 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |6 || 20 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |12 || 94 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |18 || 172 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |24 || 262 | ||
| + | |- | ||
| + | |} | ||
| + | <br> | ||
| + | ***10-6 dilute:<br> | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=20px. width=200px | Time || width=250px | Colony | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |30 || 145 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |36 || 179 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |42 || 217 | ||
| + | |- | ||
| + | |} | ||
| + | <br> | ||
| + | *Inoculate the cells for conjugation test<br> | ||
| + | 5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33<br> | ||
| + | 5ml LB/chloramphenicol + 1colony MG1655 pBAD 33<br> | ||
| + | 5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33<br> | ||
| + | 15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307<br> | ||
==Thursday, 10/16/08== | ==Thursday, 10/16/08== | ||
| - | === | + | ===Phage cloning strategy two=== |
*overnight ligation of pBluescript/insert backbone, GFP and CmR | *overnight ligation of pBluescript/insert backbone, GFP and CmR | ||
| + | ===Characterization of oriT=== | ||
| + | *Quantitatively test for oriT<br> | ||
| + | Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 1.772<br> | ||
| + | Recipient: <br> | ||
| + | overnight culture Top10 pBAD 33 OD(600nm): 2.472<br> | ||
| + | overnight culture MG1655 pBAD 33 OD(600nm): 4.412<br> | ||
| + | overnight culture DH5alpha pBAD 33 OD(600nm): 3.876<br> | ||
| + | **Centrifuge overnight culture in 1.5ml eppi for 2min at 13000rpm, 24 samples donor, 400ul/sample; 8samples Top10 recipient, 340ul/sample; 8samples MG1655 recipient, 230ul/sample; 8samples DH5alpha recipient, 260ul/sample; | ||
| + | **Wash the pellet twice with LB medium | ||
| + | **Resolve the pellet in LB medium | ||
| + | **Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid | ||
| + | **Add the washed donor suspension | ||
| + | **Vortex and resolve the pellet | ||
| + | **Centrifuge the mix for 1min at 13000rpm | ||
| + | **Resolve the pellet in 100ul LB | ||
| + | **Put membrane filter on the LB agar | ||
| + | **Pipett the suspension on membrane filter (8samples x 3tests) | ||
| + | **Incubate the plates with membrane filter at 37°C | ||
| + | **Put directly one membrane filter into 1ml LB in an 1.5ml eppi | ||
| + | **Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min) | ||
| + | **After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps. | ||
| + | **Negative control plates: | ||
| + | ***LB/Cm+Amp: <br> | ||
| + | 100ul donor overnight culture<br> | ||
| + | 100ul recipient overnight culture ( x 3) <br> | ||
| + | **Cell number determination<br> | ||
| + | ***LB/Cm: 100ul 10-6 recipient overnight culture ( x 3) | ||
| + | ***LB/Kan+Amp: 100ul 10-6 donor overnight culture<b> | ||
| + | <br> | ||
| + | *Result: | ||
| + | **Negative control: negative | ||
| + | **Colony on LB/Cm: <br> | ||
| + | Top10:179 (Titer of recipient: 1.79e9/ml) <br> | ||
| + | MG1655:242 (Titer of recipient: 2.42e9/ml) <br> | ||
| + | DH5alpha:196 (Titer of recipient: 1.96e9/ml) <br> | ||
| + | **Colony on LB/Kan+Amp: 136 (Titer of donor: 1.36e9/ml) | ||
| + | **Colony on other LB/Cm+Amp plates: | ||
| + | ***10-5 dilute: | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=20px. width=150px | Time || width=150px | Top10|| width=150px | MG1655 || width=150px | DH5alpha | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |0|| 9|| 0 || 1 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |6|| 10|| 0 || 1 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |12|| 19|| 0 || 0 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |18|| 25|| 2 || 3 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |24|| 112|| 0 || 100 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |30|| 387|| 8 || 410 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |36|| 740|| 72 || 1340* | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |42|| || 223 || 1640* | ||
| + | |- | ||
| + | |} | ||
| + | <br>'*':10-6 dilute | ||
==Friday, 10/17/08== | ==Friday, 10/17/08== | ||
| - | === | + | ===Phage cloning strategy two=== |
*transformation of overnight ligations in TOP10 | *transformation of overnight ligations in TOP10 | ||
| Line 276: | Line 749: | ||
==Saturday, 10/18/08== | ==Saturday, 10/18/08== | ||
| - | === | + | ===Phage cloning strategy two=== |
*inoculation of colonies from the transformation | *inoculation of colonies from the transformation | ||
| Line 282: | Line 755: | ||
==Sunday, 10/19/08== | ==Sunday, 10/19/08== | ||
| - | === | + | ===Phage cloning strategy two=== |
*Miniprep | *Miniprep | ||
| + | |||
| + | ===Characterization of oriT=== | ||
| + | *Inoculate the cells for conjugation test <br> | ||
| + | 5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33<br> | ||
| + | 5ml LB/chloramphenicol + 1colony MG1655 pBAD 33<br> | ||
| + | 5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33<br> | ||
| + | 15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307<br> | ||
| + | |||
| + | |||
| + | {| class="wikitable" | ||
| + | |- bgcolor=white | ||
| + | ! height=20px, width=250px | [[Team:Heidelberg/Notebook/Killing_I/Notebook/week10|<< Week 10]]|| width=500px | [[Team:Heidelberg/Notebook/Killing_I/Notebook|Overview]]|| width=250px | [[Team:Heidelberg/Notebook/Killing_I/Notebook/week12| Week 12 >> ]] | ||
| + | |-style="height:20px" | ||
| + | |} | ||
Latest revision as of 14:38, 29 October 2008


| << Week 10 | Overview | Week 12 >> |
|---|
Week 11
Contents |
Monday, 10/13/08
Proceeding of phage cloning strategy two
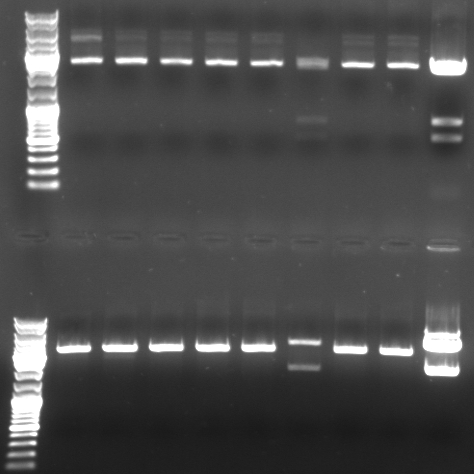
- Digestion of the miniprep 1-6,9,10 from sunday and the original pBluescript with insert
- Digestion with XbaI/XhoI (top)
- normal: 4549, 2157, 34
- new: 3945, 2898
- Digestion with SacI/SpeI (bottom)
- normal: 4549, 2157, 34
- new: 4549, 1331, 929, 34
- Gel
- lane0: dna ladder mix
- lane1: sample 1
- lane2: sample 2
- lane3: sample 3
- lane4: sample 4
- lane5: sample 5
- lane6: sample 6
- lane9: sample 9
- lane10: sample 10
- lane11: pBluescript with insert
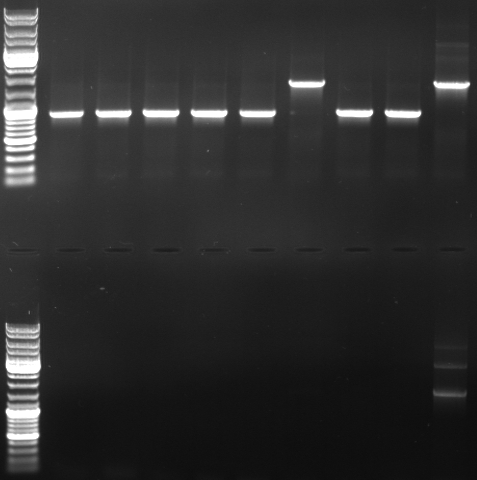
- PCR with CmR_suffix and CmR_prefix (top)
- normal: 1668bp
- new: 852bp, 919bp
- PCR with oriT_prefix and CmR_suffix (bottom)
- normal: 2150bp
- new: 1329bp
- Gel
- lane0: dna ladder mix
- lane1: sample 1
- lane2: sample 2
- lane3: sample 3
- lane4: sample 4
- lane5: sample 5
- lane6: sample 6
- lane9: sample 9
- lane10: sample 10
- lane11: pBluescript with insert
- --> we do not have a working GFP/CmR in pBlue/insert!!! --> do the ligation again (beginng from the mutagenesis pcr)
Proceeding of cloning CmR and oriT in standard plasmid
- inoculation of CmR Std. Mutagenesis PCR sample and oriT Std. colonies
Tuesday, 10/14/08
Proceeding of cloning CmR and oriT in standard plasmid
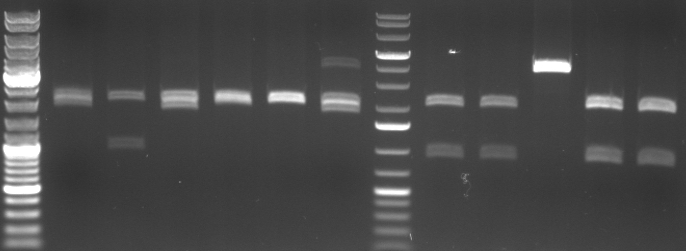
- Miniprep of 6 oriT and 5 CmR Std. samples
- digestion with EcoRI/PstI (to cut out insert of pSB1A2)
- lane0: dna ladder mix
- lane1-6: oriT 1-6
- lane7: 1kb dna ladder plus
- lane8: CmR Std. Mut 1.1.1
- lane9: CmR Std. Mut 1.1.2
- lane10: CmR Std. Mut 1.2.2
- lane11: CmR Std. Mut 2.2.1
- lane12: CmR Std. Mut 2.2.2
- expected fragments:
- oriT: 2000bp, 500bp
- CmR: 2000bp, ca. 900bp
- expected fragments:
- -->no oriT is right
- -->CmR 1.1.1, 1.1.2, 2.2.1, 2.2.2 look good
- -->sequencing of 2.2.1 and 2.2.2
- sequencing results match perfect
- Three ligations of oriT pcr product with pSB1A2 backbone (PstI, XbaI) (1:2.5, 2:5, 3:7.5 (µl, vector:insert))
- 40min at RT
- transformation, plated out on Amp plates
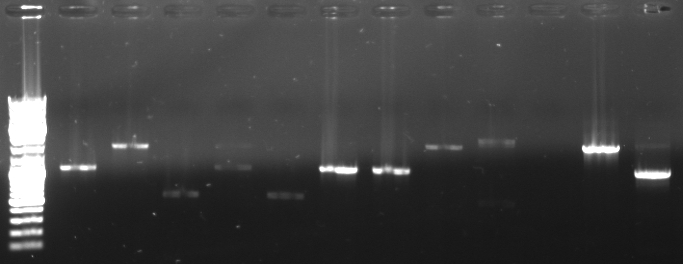
- Screening PCR of oriT agar plates
- pcr with standard plasmid primers (VF2, VR) using Taq
- 12 pcr samples (1-6 only one colony, 7-12 three colonies)
- Gel
- lane0: dna ladder mix
- lane1-12: screening sample 1-12
- expected fragment length: ca. 500-600bp
- -->sample 3 and 5 look good
- -->inoculation of overnight cultures
- -->sequencing of 3 and 5
- sequencing results match perfect
Phage cloning strategy two
- Mutagenesis PCR of pBlue with insert to remove KpnI restriction site
- using turbo Pfu
- elongation: 12,5min
- DpnI digestion 2h at 37°C
- transformation in TOP10, plated out on Cm plate
Phage cloning strategy one
- mutation of old insert in pBluescript
- pBluescript + insert was cut with BamHI --> the resulting backbone cut out of the gel and religated. With this vector 2 mutagenesis PCRs were done to eleminate the two remaining XbaI restriction sites.
- The resulting vector was digested with XbaI/XhoI to get the insert with the correct restriction sites for ligation into lambda phage
Characterization of oriT
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/kanamycin/ampicilin +glycerol stock Top10 J01103+pUB307
Wednesday, 10/15/08
Phage cloning strategy two
- inoculation of KpnI mutagenesis PCR samples --> Miniprep
- Digestion with KpnI/AgeI
- Gel
- Gel purification kit
Characterization of oriT
- Quantitatively test for oriT
Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 2.08
Recipient: overnight culture Top10 pBAD 33 OD(600nm): 2.24
- Centrifuge 350ul overnight culture in 1.5ml eppi for 2min at 13000rpm, 8samples donor, 8samples recipient
- Wash the pellet twice with LB medium
- Resolve the pellet in 350ul LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 100ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (8samples)
- Incubate the plates with membrane filter at 37°C
- Put directly one membrane filter into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min)
- After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps.
- Negative control plates:
- LB/Cm+Amp:
100ul donor overnight culture
100ul recipient overnight culture
- Cell number determination
- LB/Cm: 100ul 10-6 recipient overnight culture
- LB/Kan+Amp: 100ul 10-6 donor overnight culture
- Cell number determination
- Result:
- Negative control: negative
- Colony on LB/Cm: 238 (Titer of recipient: 2.38e9/ml)
- Colony on LB/Kan+Amp: 99 (Titer of donor: 0.99e9/ml)
- Colony on other LB/Cm+Amp plates:
- 10-5 dilute:
- 10-5 dilute:
| Time | Colony |
|---|---|
| 0 | 9 |
| 6 | 20 |
| 12 | 94 |
| 18 | 172 |
| 24 | 262 |
- 10-6 dilute:
- 10-6 dilute:
| Time | Colony |
|---|---|
| 30 | 145 |
| 36 | 179 |
| 42 | 217 |
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/chloramphenicol + 1colony MG1655 pBAD 33
5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33
15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307
Thursday, 10/16/08
Phage cloning strategy two
- overnight ligation of pBluescript/insert backbone, GFP and CmR
Characterization of oriT
- Quantitatively test for oriT
Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 1.772
Recipient:
overnight culture Top10 pBAD 33 OD(600nm): 2.472
overnight culture MG1655 pBAD 33 OD(600nm): 4.412
overnight culture DH5alpha pBAD 33 OD(600nm): 3.876
- Centrifuge overnight culture in 1.5ml eppi for 2min at 13000rpm, 24 samples donor, 400ul/sample; 8samples Top10 recipient, 340ul/sample; 8samples MG1655 recipient, 230ul/sample; 8samples DH5alpha recipient, 260ul/sample;
- Wash the pellet twice with LB medium
- Resolve the pellet in LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 100ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (8samples x 3tests)
- Incubate the plates with membrane filter at 37°C
- Put directly one membrane filter into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min)
- After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps.
- Negative control plates:
- LB/Cm+Amp:
- LB/Cm+Amp:
100ul donor overnight culture
100ul recipient overnight culture ( x 3)
- Cell number determination
- LB/Cm: 100ul 10-6 recipient overnight culture ( x 3)
- LB/Kan+Amp: 100ul 10-6 donor overnight culture
- Cell number determination
- Result:
- Negative control: negative
- Colony on LB/Cm:
Top10:179 (Titer of recipient: 1.79e9/ml)
MG1655:242 (Titer of recipient: 2.42e9/ml)
DH5alpha:196 (Titer of recipient: 1.96e9/ml)
- Colony on LB/Kan+Amp: 136 (Titer of donor: 1.36e9/ml)
- Colony on other LB/Cm+Amp plates:
- 10-5 dilute:
| Time | Top10 | MG1655 | DH5alpha |
|---|---|---|---|
| 0 | 9 | 0 | 1 |
| 6 | 10 | 0 | 1 |
| 12 | 19 | 0 | 0 |
| 18 | 25 | 2 | 3 |
| 24 | 112 | 0 | 100 |
| 30 | 387 | 8 | 410 |
| 36 | 740 | 72 | 1340* |
| 42 | 223 | 1640* |
'*':10-6 dilute
Friday, 10/17/08
Phage cloning strategy two
- transformation of overnight ligations in TOP10
Saturday, 10/18/08
Phage cloning strategy two
- inoculation of colonies from the transformation
Sunday, 10/19/08
Phage cloning strategy two
- Miniprep
Characterization of oriT
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/chloramphenicol + 1colony MG1655 pBAD 33
5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33
15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307
| << Week 10 | Overview | Week 12 >> |
|---|
 "
"