Team:Heidelberg/Notebook/Killing I/Notebook/week11
From 2008.igem.org


| << Week 10 | Overview | Week 12 >> |
|---|
Week 11
Contents |
Monday, 10/13/08
Proceeding of phage cloning strategy two
- Digestion of the miniprep 1-6,9,10 from sunday and the original pBluescript with insert
- Digestion with XbaI/XhoI (top)
- normal: 4549, 2157, 34
- new: 3945, 2898
- Digestion with SacI/SpeI (bottom)
- normal: 4549, 2157, 34
- new: 4549, 1331, 929, 34
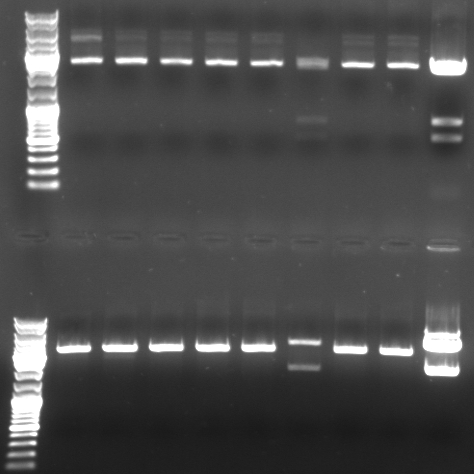
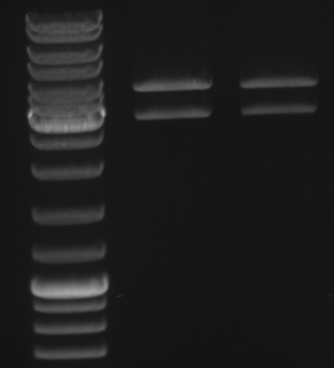
- Gel
- lane0: dna ladder mix
- lane1: sample 1
- lane2: sample 2
- lane3: sample 3
- lane4: sample 4
- lane5: sample 5
- lane6: sample 6
- lane9: sample 9
- lane10: sample 10
- lane11: pBluescript with insert
- PCR with CmR_suffix and CmR_prefix (top)
- normal: 1668bp
- new: 852bp, 919bp
- PCR with oriT_prefix and CmR_suffix (bottom)
- normal: 2150bp
- new: 1329bp
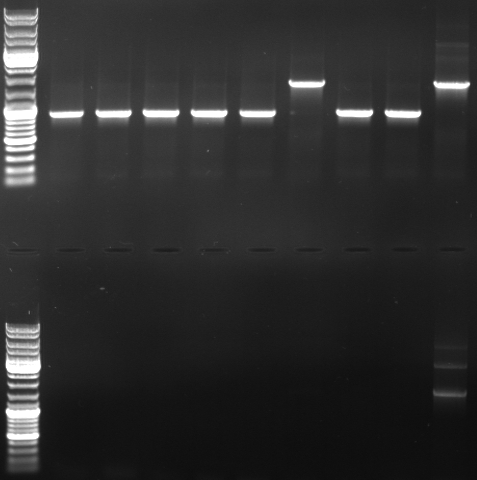
- Gel
- lane0: dna ladder mix
- lane1: sample 1
- lane2: sample 2
- lane3: sample 3
- lane4: sample 4
- lane5: sample 5
- lane6: sample 6
- lane9: sample 9
- lane10: sample 10
- lane11: pBluescript with insert
- --> we do not have a working GFP/CmR in pBlue/insert!!! --> do the ligation again (beginng from the mutagenesis pcr)
Proceeding of cloning CmR and oriT in standard plasmid
- inoculation of CmR Std. Mutagenesis PCR sample and oriT Std. colonies
Tuesday, 10/14/08
Proceeding of cloning CmR and oriT in standard plasmid
- Miniprep of 6 oriT and 5 CmR Std. samples
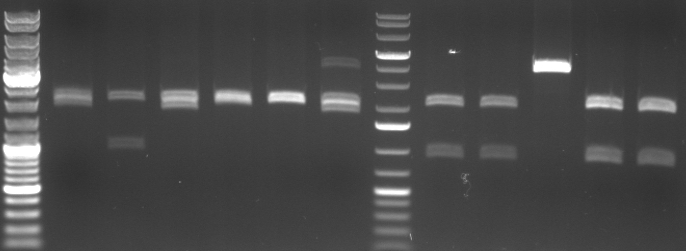
- digestion with EcoRI/PstI (to cut out insert of pSB1A2)
- lane0: dna ladder mix
- lane1-6: oriT 1-6
- lane7: 1kb dna ladder plus
- lane8: CmR Std. Mut 1.1.1
- lane9: CmR Std. Mut 1.1.2
- lane10: CmR Std. Mut 1.2.2
- lane11: CmR Std. Mut 2.2.1
- lane12: CmR Std. Mut 2.2.2
- expected fragments:
- oriT: 2000bp, 500bp
- CmR: 2000bp, ca. 900bp
- expected fragments:
- -->no oriT is right
- -->CmR 1.1.1, 1.1.2, 2.2.1, 2.2.2 look good
- -->sequencing of 2.2.1 and 2.2.2
- sequencing results match perfect
- Three ligations of oriT pcr product with pSB1A2 backbone (PstI, XbaI) (1:2.5, 2:5, 3:7.5 (µl, vector:insert))
- 40min at RT
- transformation, plated out on Amp plates
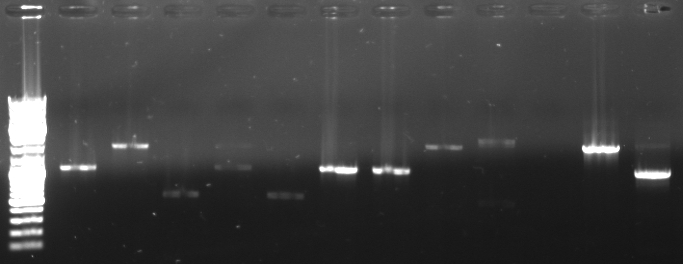
- Screening PCR of oriT agar plates
- pcr with standard plasmid primers (VF2, VR) using Taq
- 12 pcr samples (1-6 only one colony, 7-12 three colonies)
- Gel
- lane0: dna ladder mix
- lane1-12: screening sample 1-12
- expected fragment length: ca. 500-600bp
- -->sample 3 and 5 look good
- -->inoculation of overnight cultures
- -->sequencing of 3 and 5
- sequencing results match perfect
Phage cloning strategy two
- Mutagenesis PCR of pBlue with insert to remove KpnI restriction site
- using turbo Pfu
- elongation: 12,5min
- DpnI digestion 2h at 37°C
- transformation in TOP10, plated out on Cm plate
Phage cloning strategy one
- mutation of old insert in pBluescript
- pBluescript + insert was cut with BamHI --> the resulting backbone cut out of the gel and religated. With this vector 2 mutagenesis PCRs were done to eleminate the two remaining XbaI restriction sites.
- The resulting vector was digested with XbaI/XhoI to get the insert with the correct restriction sites for ligation into lambda phage
Characterization of oriT
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/kanamycin/ampicilin +glycerol stock Top10 J01103+pUB307
Wednesday, 10/15/08
Phage cloning strategy two
- inoculation of KpnI mutagenesis PCR samples --> Miniprep
- Digestion with KpnI/AgeI
- Gel
- Gel purification kit
Characterization of oriT
- Quantitatively test for oriT
Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 2.08
Recipient: overnight culture Top10 pBAD 33 OD(600nm): 2.24
- Centrifuge 350ul overnight culture in 1.5ml eppi for 2min at 13000rpm, 8samples donor, 8samples recipient
- Wash the pellet twice with LB medium
- Resolve the pellet in 350ul LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 100ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (8samples)
- Incubate the plates with membrane filter at 37°C
- Put directly one membrane filter into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min)
- After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps.
- Negative control plates:
- LB/Cm+Amp:
100ul donor overnight culture
100ul recipient overnight culture
- Cell number determination
- LB/Cm: 100ul 10-6 recipient overnight culture
- LB/Kan+Amp: 100ul 10-6 donor overnight culture
- Cell number determination
- Result:
- Negative control: negative
- Colony on LB/Cm: 238 (Titer of recipient: 2.38e9/ml)
- Colony on LB/Kan+Amp: 99 (Titer of donor: 0.99e9/ml)
- Colony on other LB/Cm+Amp plates:
- 10-5 dilute:
- 10-5 dilute:
Time Colony
0 9
6 20
12 94
18 172
24 262
- 10-6 dilute:
- 10-6 dilute:
Time Colony
30 145
36 179
42 217
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/chloramphenicol + 1colony MG1655 pBAD 33
5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33
15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307
Thursday, 10/16/08
Phage cloning strategy two
- overnight ligation of pBluescript/insert backbone, GFP and CmR
Characterization of oriT
- Quantitatively test for oriT
Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 1.772
Recipient:
overnight culture Top10 pBAD 33 OD(600nm): 2.472
overnight culture MG1655 pBAD 33 OD(600nm): 4.412
overnight culture DH5alpha pBAD 33 OD(600nm): 3.876
- Centrifuge overnight culture in 1.5ml eppi for 2min at 13000rpm, 24 samples donor, 400ul/sample; 8samples Top10 recipient, 340ul/sample; 8samples MG1655 recipient, 230ul/sample; 8samples DH5alpha recipient, 260ul/sample;
- Wash the pellet twice with LB medium
- Resolve the pellet in LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 100ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (8samples x 3tests)
- Incubate the plates with membrane filter at 37°C
- Put directly one membrane filter into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min)
- After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps.
- Negative control plates:
- LB/Cm+Amp:
- LB/Cm+Amp:
100ul donor overnight culture
100ul recipient overnight culture ( x 3)
- Cell number determination
- LB/Cm: 100ul 10-6 recipient overnight culture ( x 3)
- LB/Kan+Amp: 100ul 10-6 donor overnight culture
- Cell number determination
- Result:
- Negative control: negative
- Colony on LB/Cm:
Top10:179 (Titer of recipient: 1.79e9/ml)
MG1655:242 (Titer of recipient: 2.42e9/ml)
DH5alpha:196 (Titer of recipient: 1.96e9/ml)
- Colony on LB/Kan+Amp: 136 (Titer of donor: 1.36e9/ml)
- Colony on other LB/Cm+Amp plates:
- 10-5 dilute:
Time Top10 MG1655 DH5alpha
0 9 0 1
6 10 0 1
12 19 0 0
18 25 2 3
24 112 0 100
30 387 8 410
36 740 72 1340x
42 223 1640x
x:10-6 dilute
Friday, 10/17/08
Phage cloning strategy two
- transformation of overnight ligations in TOP10
Saturday, 10/18/08
Phage cloning strategy two
- inoculation of colonies from the transformation
Sunday, 10/19/08
Phage cloning strategy two
- Miniprep
| << Week 10 | Overview | Week 12 >> |
|---|
Characterization of oriT
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/chloramphenicol + 1colony MG1655 pBAD 33
5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33
15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307
 "
"