Team:Heidelberg/Notebook/Visualization/Methods
From 2008.igem.org
(Difference between revisions)
(→Swarm Assays) |
(→Swarm Assays) |
||
| Line 501: | Line 501: | ||
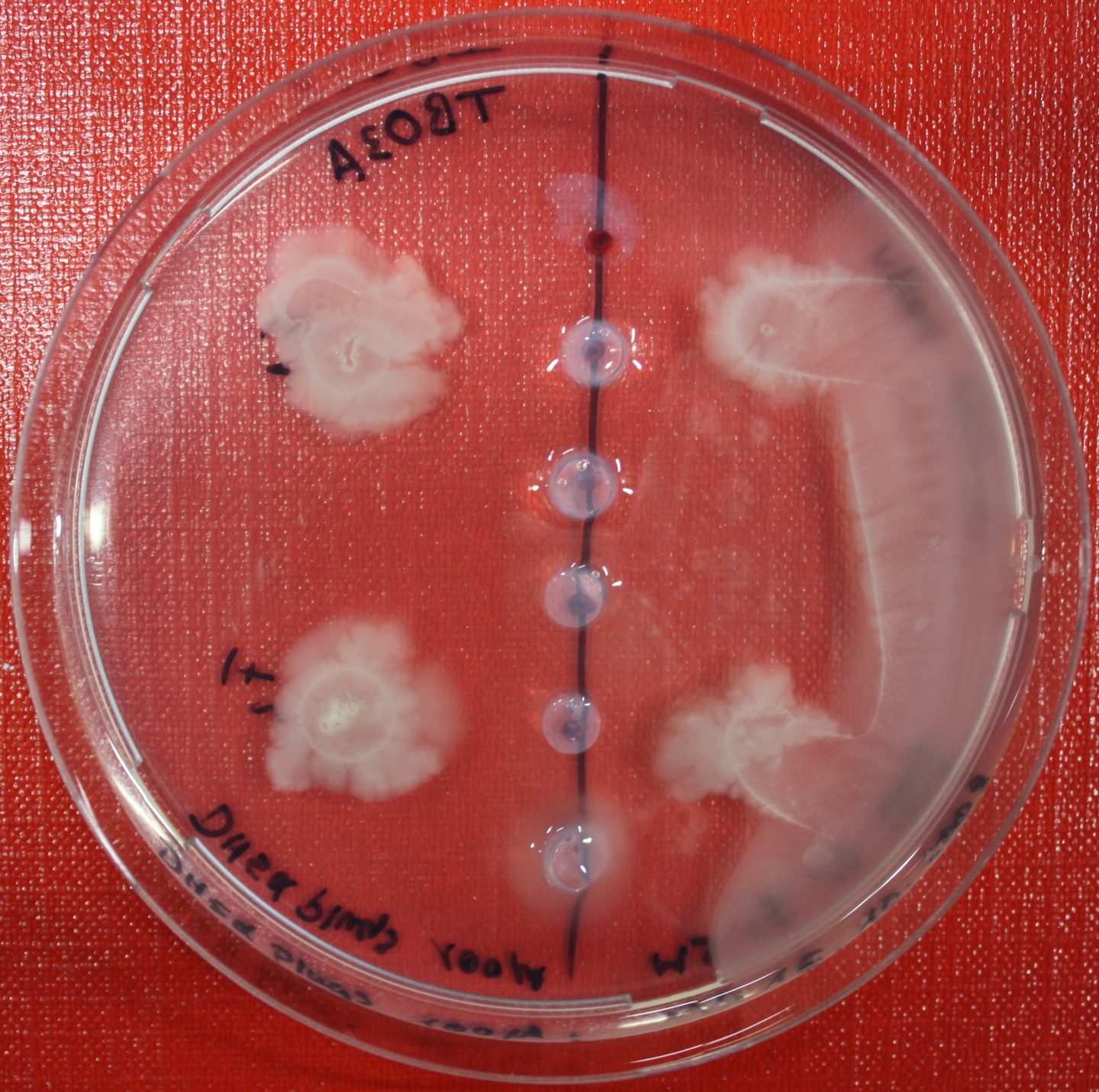
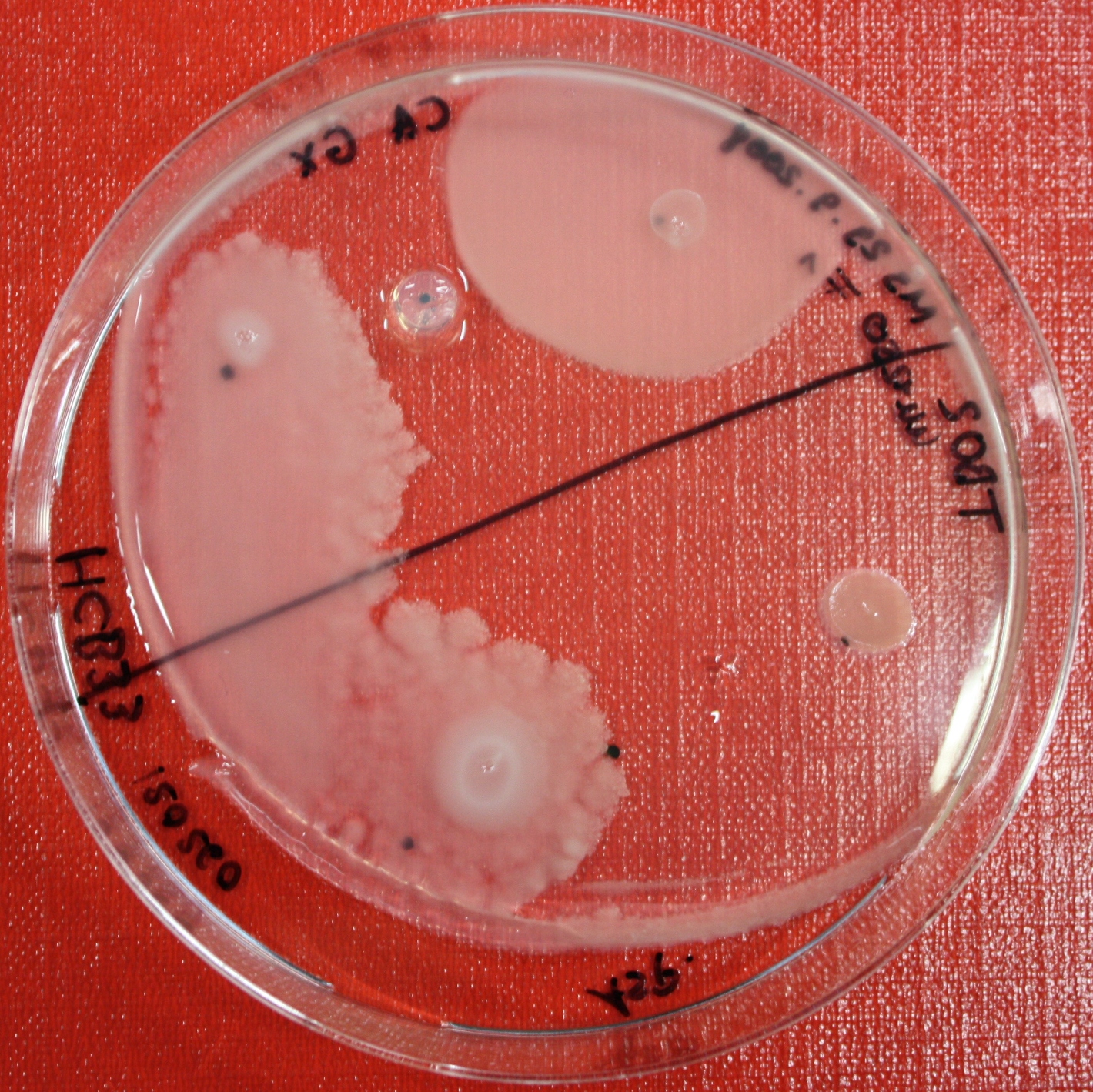
| - | [[Image:SwarmA_CA_line.jpg|left|thumb|400px| | + | [[Image:SwarmA_CA_line.jpg|left|thumb|400px|Center attractant plug line]][[Image:SwarmA_CA_spot.jpg|right|thumb|400px|Spotted attractants]] |
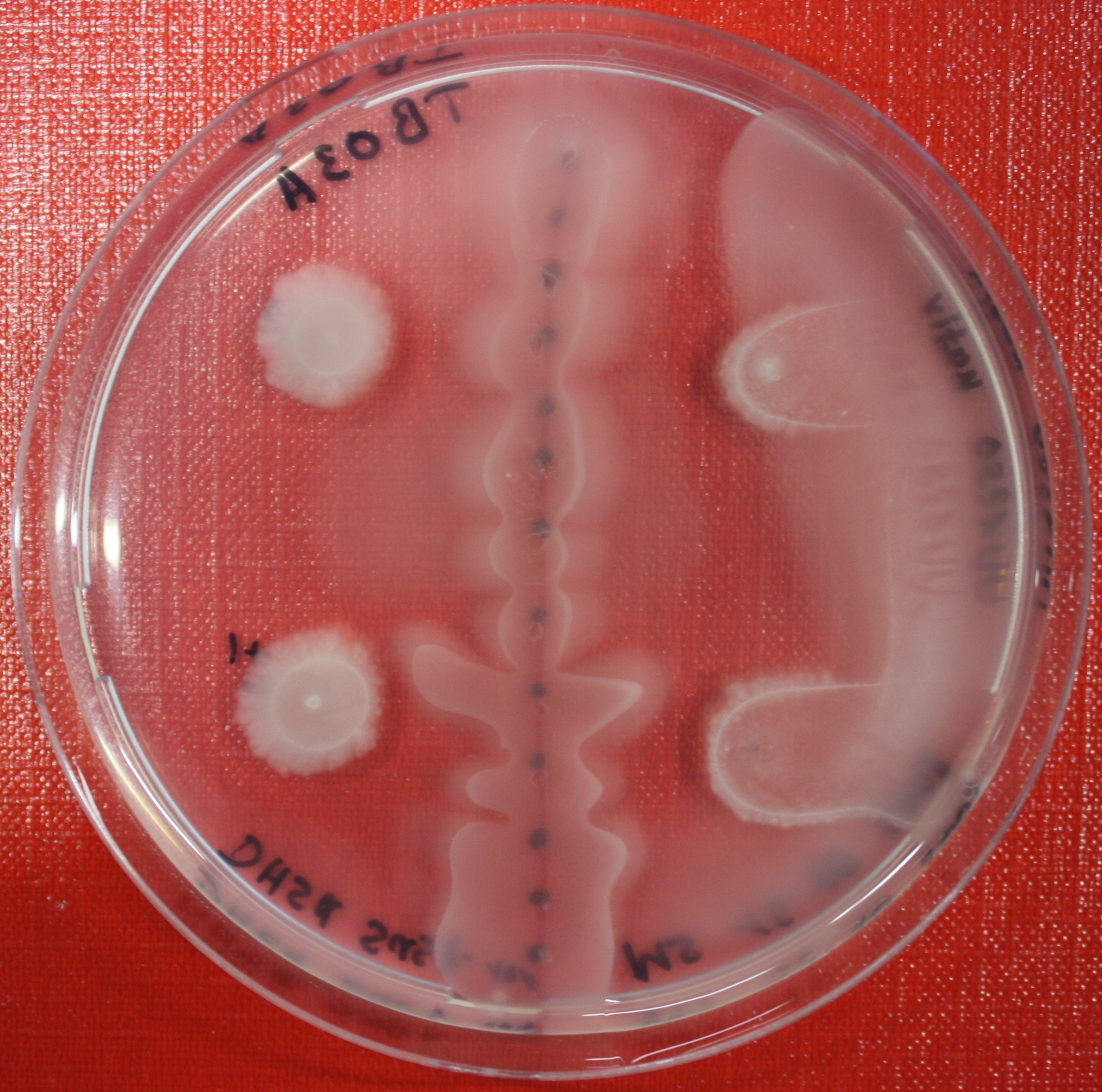
| - | [[Image:SwarmA_fluid_attractant.jpg|left|thumb|400px| | + | [[Image:SwarmA_fluid_attractant.jpg|left|thumb|400px|Center fluid attractant line]] [[Image:SwarmA_alternative_chamber.jpg|left|thumb|400px|This picture is showing an alternative chamber]] |
Revision as of 20:32, 28 October 2008


Contents |
Microscope
Small chamber
chemotactical activity
Large chamber
computer-assisted analysis
Swarm Assays
PROTOCOL FOR SWARM-PLATE CHEMOTAXIS AGAINST A POSSIBLE ATTRACTANT:
This protocol was created using petri-dishes. But every other chamber could be used as well.
- Step 1: Make sure you have everything ready to get started. You need:
- Petri-dishes. !!! sign your petri-dishes before You pour in the media. Also mark the spots where you want to set the attractant or attractant line and the bacteria. !!!
- 50 ml falcons
- 25 ml media containig 0.2%-0.3% Agar for every petri-dish.
- samples of the appropriate attractants. The concentration depends on several substance depending attributes.
- Other substances like antibiotics (Amp, Kan, Cm) or inducer (IPTG, Arabinose)
- Step 2: Your agar-media solution should be clear and the agar completely solved. If you want to use attractant plugs1 or other non fluid attractants, go first through step 5 then back through 2,3,4 skip 5, further with 6 and so on...
- Step 3: Take Take a 50 ml falcon pour 25 ml of your agar containing media in it and add antibiotics or inducing substances. If no extra substances are needed just skip this step.
- Step 4: Let your media polimerize. At least let it rest for 15/20 min.
- Step 5: Then add the attractant. e.g.: a casamino acid solution
- Step 6:
- Put the whole plate into a 4°C fridge for 12-16 hours to allow the attractant to build up a gradient.
- Make an overnight culture of the strains you want to test, use TB media and add inducing substances if something has to be induced.
Next day:
- Step 7: Take your overnight culture. It should be at an OD of 0.4-0.6. Then take 1 ml of the bacteria suspension and centrifuge it for 10 minutes at 4,000 rpm. Resolve the pellet at 100 µl of the media you are using in the petri-dish.
- Step 8: Place 3-6 µl of your purrified bacteria at the premarked spots on the petri-dish sample.
- Step 9: Put the whole plate into the incubator at ~30°C and take a look at your plates after 24, 48 and 72 hours. Put the plates into a plastic bag like the small ones for the bench trash to protect the plates from running dry to fast. Only move the plates if necessary.
1: Attractant plugs can be created by adding agar to the attractant solution.
 "
"