Team:Hawaii/Notebook/2008-07-18
From 2008.igem.org
(Difference between revisions)
m (updating notebook) |
(→Wetlab work) |
||
| (7 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
:<strong>Margaret</strong> | :<strong>Margaret</strong> | ||
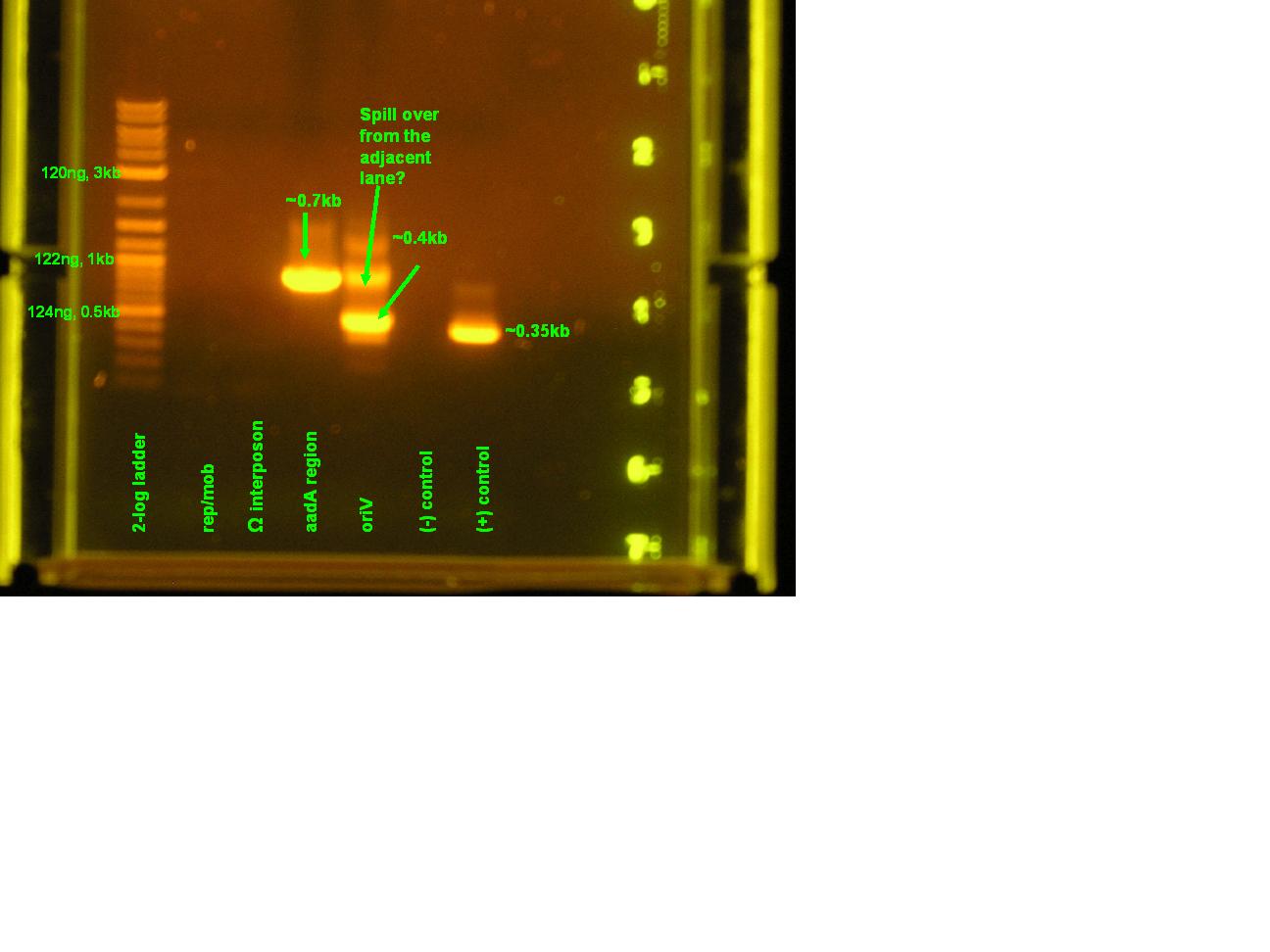
| - | :* | + | :*for details concerning the running conditions, please look [[Team:Hawaii/PCR Amplification of pRL1383a|here]]. |
| - | :* | + | :*Ran a 0.8%gel at 95V. [[Image:PCR_7_18_08.jpg|right|thumb|300px|Please refer to the annotated picture.]] |
===Extraction of Biobricks and Transformation in DB3.1=== | ===Extraction of Biobricks and Transformation in DB3.1=== | ||
:<strong>Margaret</strong> | :<strong>Margaret</strong> | ||
| + | :*streaked plates on Amp100 plates | ||
| + | :*for details please look [[Team:Hawaii/Initial E. Coli Transformation|here]]. | ||
| + | |||
| + | === Sequencing=== | ||
| + | :<strong>Grace</strong> | ||
| + | |||
| + | :* Redid PCR reactions for GFP fusion, BB-pRL1383a, B0015, J04430, R0010 | ||
| + | ::* Gel OK this time (95V for 50 min; ladder resolved, loading dye didn't run funny) | ||
| + | ::* Incorrect bands still for B0015, J04430, R0010; Krystle will redo the plasmid preps for these | ||
| + | ::* Correct bands for GFP fusion and BB-pRL1383a. BB-pRL1383a band faint -- why? | ||
| + | :* Treated PCR products w/ [[Team:Hawaii/Protocols/ExoSAP|ExoSAP]] | ||
| + | :* Determined DNA concentrations using nanodrop spectrometer (measured twice) | ||
| + | {| border="1" align="center" | ||
| + | |+''' DNA concentrations of PCR products''' | ||
| + | !PCR Sample | ||
| + | !Concentration (1st measurement) | ||
| + | !Concentration (2nd measurement) | ||
| + | |- | ||
| + | | align="center"|nir | ||
| + | | align="center"|1015 ng/μl || align="center"|1010 ng/μl | ||
| + | |- | ||
| + | | align="center"|slr2016-1 | ||
| + | |align="center"|1295 ng/μl | ||
| + | |align="center"|1107 ng/μl | ||
| + | |- | ||
| + | | align="center"|slr2016-2 | ||
| + | | align="center"|1518 ng/μl|| align="center"|1266 ng/μl | ||
| + | |- | ||
| + | | align="center"|pilA | ||
| + | | align="center"|1312 ng/μl|| align="center"|1330 ng/μl | ||
| + | |- | ||
| + | | align="center"| B0024 | ||
| + | | align="center"|1546 ng/μl|| align="center"|1474 ng/μl | ||
| + | |- | ||
| + | | align="center"|B0034 | ||
| + | | align="center"|1537 ng/μl|| align="center"|1386 ng/μl | ||
| + | |- | ||
| + | | align="center"|C0012 | ||
| + | | align="center"|2130 ng/μl|| align="center"|1530 ng/μl | ||
| + | |- | ||
| + | | align="center"|E0040 | ||
| + | | align="center"|1502 ng/μl|| align="center"|1236 ng/μl | ||
| + | |- | ||
| + | | align="center"|J33207 | ||
| + | | align="center"|1454 ng/μl|| align="center"|1223 ng/μl | ||
| + | |- | ||
| + | | align="center"|BB-pRL1383a | ||
| + | | align="center"|2279 ng/μl|| align="center"|2129 ng/μl | ||
| + | |- | ||
| + | | align="center"|GFP fusion | ||
| + | | align="center"|1660 ng/μl|| align="center"|1714 ng/μl | ||
| + | |} | ||
| + | :* Sent 22 samples to CORE Hawaii for sequencing | ||
| + | |||
| + | ===Media Making=== | ||
| + | <strong>Margaret, Krystle (thanks for the help!)</strong> | ||
| + | :* Made one sleeve of Amp100 plates. | ||
| + | |||
| + | ===Plasmid Prep of GFP fusion Brick, R0010, J3340=== | ||
| + | :<strong>Krystle</strong> | ||
| + | |||
| + | :*did a large scale (75 ml) plasmid prep for the GFP fusion brick | ||
| + | :*Performed 3 mini preps each of the BioBrick parts | ||
== Drylab Work == | == Drylab Work == | ||
| - | |||
| - | |||
| - | : | + | ===[[Team:Hawaii/Project|Project Description (Abstract]])=== |
| - | : | + | :<strong> Grace, Krystle, Margaret</strong> |
| + | :* Wrote description of project (abstract) | ||
= Discussion = | = Discussion = | ||
Latest revision as of 04:08, 22 July 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
PCR of pRL1383a Parts
- Margaret
- for details concerning the running conditions, please look here.
- Ran a 0.8%gel at 95V.
Extraction of Biobricks and Transformation in DB3.1
- Margaret
- streaked plates on Amp100 plates
- for details please look here.
Sequencing
- Grace
- Redid PCR reactions for GFP fusion, BB-pRL1383a, B0015, J04430, R0010
- Gel OK this time (95V for 50 min; ladder resolved, loading dye didn't run funny)
- Incorrect bands still for B0015, J04430, R0010; Krystle will redo the plasmid preps for these
- Correct bands for GFP fusion and BB-pRL1383a. BB-pRL1383a band faint -- why?
- Treated PCR products w/ ExoSAP
- Determined DNA concentrations using nanodrop spectrometer (measured twice)
| PCR Sample | Concentration (1st measurement) | Concentration (2nd measurement) |
|---|---|---|
| nir | 1015 ng/μl | 1010 ng/μl |
| slr2016-1 | 1295 ng/μl | 1107 ng/μl |
| slr2016-2 | 1518 ng/μl | 1266 ng/μl |
| pilA | 1312 ng/μl | 1330 ng/μl |
| B0024 | 1546 ng/μl | 1474 ng/μl |
| B0034 | 1537 ng/μl | 1386 ng/μl |
| C0012 | 2130 ng/μl | 1530 ng/μl |
| E0040 | 1502 ng/μl | 1236 ng/μl |
| J33207 | 1454 ng/μl | 1223 ng/μl |
| BB-pRL1383a | 2279 ng/μl | 2129 ng/μl |
| GFP fusion | 1660 ng/μl | 1714 ng/μl |
- Sent 22 samples to CORE Hawaii for sequencing
Media Making
Margaret, Krystle (thanks for the help!)
- Made one sleeve of Amp100 plates.
Plasmid Prep of GFP fusion Brick, R0010, J3340
- Krystle
- did a large scale (75 ml) plasmid prep for the GFP fusion brick
- Performed 3 mini preps each of the BioBrick parts
Drylab Work
Project Description (Abstract)
- Grace, Krystle, Margaret
- Wrote description of project (abstract)
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"