|
Home
The Team
Project Report
Parts
Modeling
Notebook
Safety
CoLABoration
|
_project report
Introduction:
This year´s main project is the attempt to create an "artificial receptor-system", featuring extra- and intracellular modules as well as suitable transmembrane regions.
The intracellular domaine of our receptor-device is build by halves of split reporter-proteins that can reassemble and will then produce readable output, e. g. fluorescence.
Each one of these protein-halves is connected to its extracellular domaine by a single-span transmembrane-helix.
The extracellular or detecting domaine consists of a protein or peptide with the ability to bind a certain molecule.
Now, if a system with two matching receptors is presented these molecules in a strict, pairwise spatial arrangement, the receptor-devices are brought together,
the split reporter-protein reassembles inside the cell and the output can be detected.
We employ so-called "Origami-DNA" to create the exactly defined molecule-patterns that are needed to activate our receptors.
One of the main inspirations that lead to the idea of creating a synthetic receptor-like fusion protein is based on an immunologic study on the signaling pathway of the T-Cell-Receptor (TCR) that has been performed by Wolfgang Schamel at the Max-Planck-Institute for immunology, Freiburg[1].
In this study he used modified TCRs with Fab-Fragment-singlechains of Anti-NIP –Antibodies fused to their ß-domaines by a flexible linker that would present them on the cell´s surface.
This modification would allow to investigate the influence of receptor-clustering on the intensity of the cell-signaling. It could been shown that there is a relation between the clustering of the antigen and, thus, of the receptors by presenting various peptides with certain amounts and arrangements of NIP-molecules as stimulus.
Anyway, this experiment was restricted by the one-dimensionality of the antigen-fused peptides; at this point, the Origami-DNA comes into play:
Paul Rothemund had discovered that it is possible to shape M13-Phage single-strand-DNA simply adding oligonucleotides that would work as „brackets“ when complementing the long single-strand. In this way, one can generate DNA-squares of a certain size with „nods“ at certain distances.
One member of our team, Daniel Hautzinger, has recently finished his diploma-thesis on Origami-DNA and the possibilities of generating patterns on these square surfaces by modifying the Oligo-nucleotides that build up the nod-points.
As the antigen NIP can as well be fused to these oligos, it was now possible to present strictly defined two-dimensional antigen-patterns to T-Cells carrying the modified receptors mentioned above.
This, again, made us come up with the idea of a transmembrane-fusion-protein that could be spatially arranged from outside the cell by the pattern on the Origami-DNA-surface.
Of course, the first extracellular domaine we had in mind was the anti-NIP-singlechain Schamel had used with his receptors. The first intracellular domaines should consist of the split-lactamase-halfes we designed as parts for last year´s iGEM, as this enzyme´s activity can be regained by complementation of the halves and detected by a fluorescent substrate.
Now, we were looking for a single-span-transmembrane-protein; as the domaines of the Epidermal-Growth-Factor Receptor are well known, we chose to employ it´s transmembrane-helix and the signal-peptide mediating the construct´s insertion into the membrane.
Further modules we had in mind were an Anti-Fluorescein-singlechain and a fluorescein-binding variety of Lipocalin by Arne Skerra as extracellular „detectors“ as well as the complementing halves of each one of the split-fluorophores „Cerulean“ (cyan) and „Venus“ (yellow) as intracellular „reporters“. These split-fluorophores feature cross-compatibility between the N- and C-terminal halves (green fluorescence), enabling us to generate three different „outputs“ (yellow, blue, green) with only two molecules (NIP, FluA) building up the „input-pattern“ on the Origami-DNA-surface.
Subprojects:
DNA-Origami
Cloning Strategy
Cell Culture
Transfection
Calcium Imaging
Results:
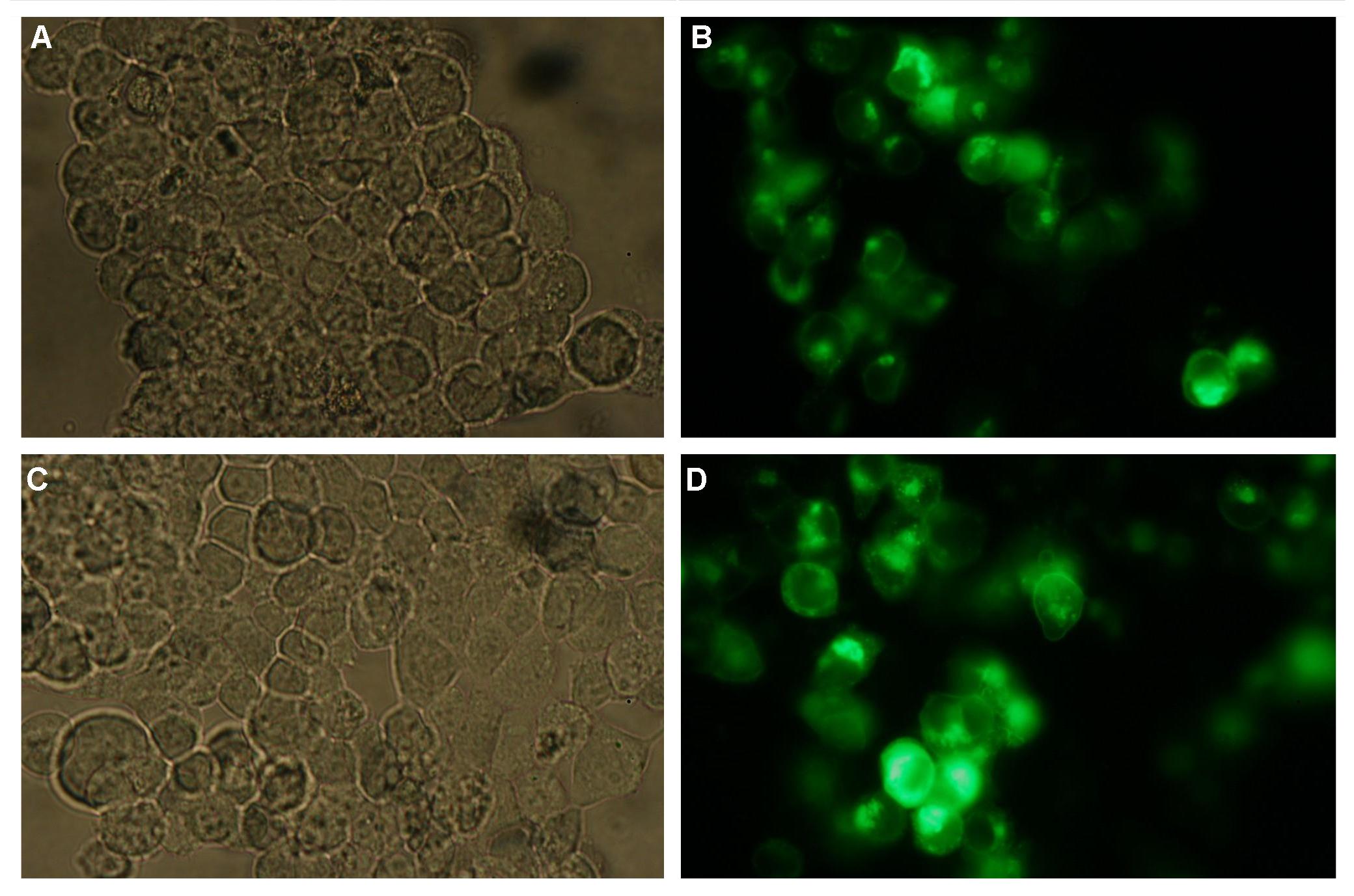 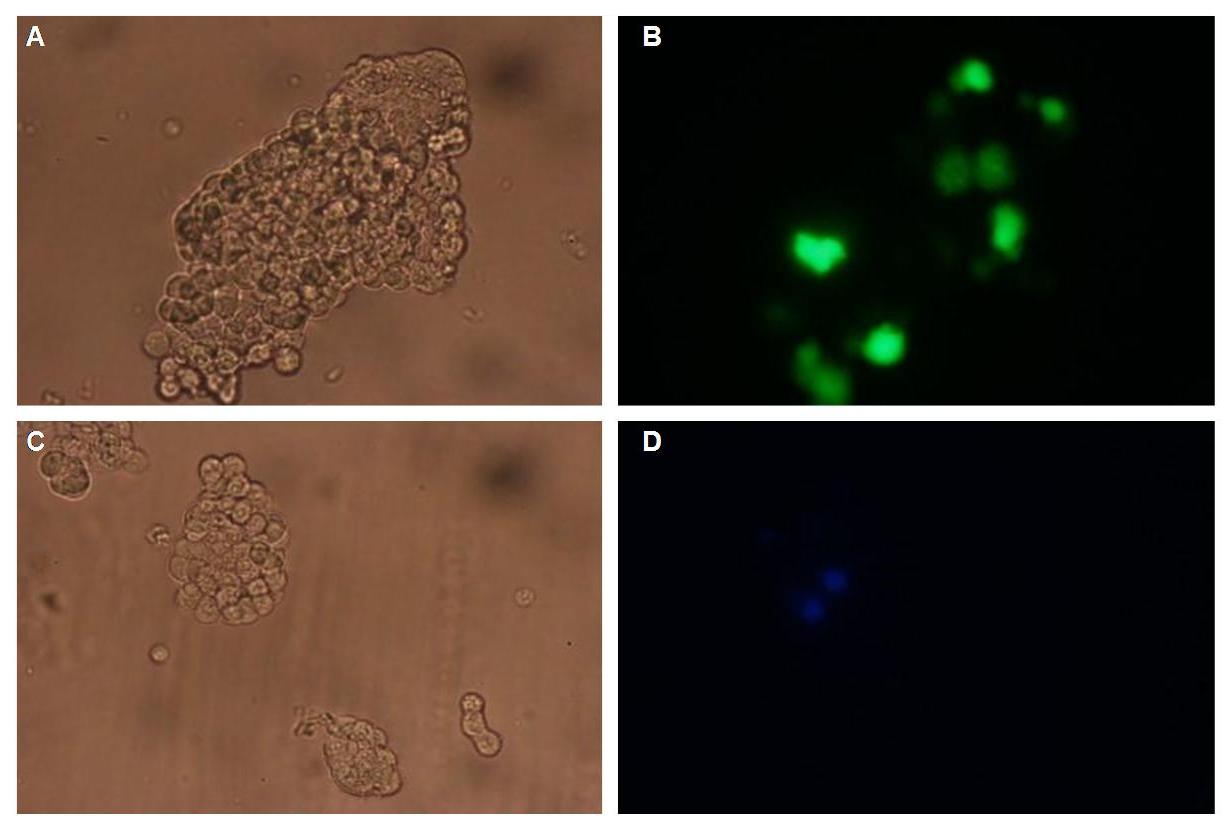 Fig.1: left: Transfection of 293T-Cells with one of our membrane-localized constructs (Part Bba_K157032-YFP),
Fig.1: left: Transfection of 293T-Cells with one of our membrane-localized constructs (Part Bba_K157032-YFP),
right: cytosolic YFP (CMV-Promotor)
Discussion:
Literature:
Split-fluorophores:
-Chang-Deng Hu, Yurii Chinenov, Tom K. Kerppola: ”Visualization of Interactions among bZIP and Rel Family Proteins in Living Cells Using Bimolecular Fluorescence Complementation”, Molecular Cell, Vol. 9, 789–798, April, 2002
-Chang Deng Hu, Tom K. Kerppola: “Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis”, Nat Biotechnol. 2003 May; 21(5):539-545 (doi:10. 1038/nbt816)
-Tom K. Kerppola: “Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells”, Nat Protoc. 2006;1(3):1278-1286 (doi:10.1038/nprot.2006.201)
-Nagai, T. et al. “A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications” J. Biol. Chem. 276, 29188-29194, 2001
-Roger Y. Tsien et al. „Creating new fluorescent probes for cell biology“, Nature Biotechnology Reviews, Vol. 3, 906-918, 2002
LipocalinFluA:
-Gerald Beste, Frank S. Schmidt, Thomas Stibora and A. Skerra: “Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold“,Proc. Natl. Acad. Sci. USA Vol. 96, pp. 1898–1903, March 1999 Biochemistry
-Ingo P. Korndörfer, Gerald Beste and A. Skerra: “Crystallographic Analysis of an “Anticalin” With Tailored Specificity for Fluorescein Reveals High Structural Plasticity of the Lipocalin Loop Region”, PROTEINS: Structure, Function, and Bioinformatics 53:121–129 (2003)
DNA-Origami:
Paul W. K. Rothemund: Nature 440, 297-302 (16 March 2006)
Antibody B1-8:
-Ana Cumano and Klaus Rajewski: “Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP”, The EMBO Journal vol. 5 no.10 pp. 2459-2468, 1986
-D. Allen, T. Simon, F. Sablitzky, K. Rajewski and A. Cumano: “Antibody engineering for the analysis of affinity maturation of an anti-hapten response”, The EMBO Journal vol. 7 no.7 pp. 1995-2001, 1988
|
 "
"


