Team:Freiburg/Project
From 2008.igem.org
m |
m |
||
| Line 24: | Line 24: | ||
Of course, the first extracellular domaine we had in mind was the anti-NIP-singlechain Schamel had used with his receptors. The first intracellular domaines should consist of the split-lactamase-halfes we designed as parts for last year´s iGEM, as this enzyme´s activity can be regained by complementation of the halves and detected by a fluorescent substrate. | Of course, the first extracellular domaine we had in mind was the anti-NIP-singlechain Schamel had used with his receptors. The first intracellular domaines should consist of the split-lactamase-halfes we designed as parts for last year´s iGEM, as this enzyme´s activity can be regained by complementation of the halves and detected by a fluorescent substrate. | ||
Now, we were looking for a single-span-transmembrane-protein; as the domaines of the Epidermal-Growth-Factor Receptor are well known, we chose to employ it´s transmembrane-helix and the signal-peptide mediating the construct´s insertion into the membrane.<br> | Now, we were looking for a single-span-transmembrane-protein; as the domaines of the Epidermal-Growth-Factor Receptor are well known, we chose to employ it´s transmembrane-helix and the signal-peptide mediating the construct´s insertion into the membrane.<br> | ||
| - | Further modules we had in mind were an Anti-Fluorescein-singlechain and a fluorescein-binding variety of Lipocalin by Arne Skerra as extracellular „detectors“ as well as the complementing halves of each one of the split-fluorophores „Cerulean“ (cyan) and „Venus“ (yellow) as intracellular „reporters“. These split-fluorophores feature cross-compatibility between the N- and C-terminal halves (green fluorescence), enabling | + | Further modules we had in mind were an Anti-Fluorescein-singlechain and a fluorescein-binding variety of Lipocalin by Arne Skerra as extracellular „detectors“ as well as the complementing halves of each one of the split-fluorophores „Cerulean“ (cyan) and „Venus“ (yellow) as intracellular „reporters“. These split-fluorophores feature cross-compatibility between the N- and C-terminal halves (green fluorescence), enabling our system to generate three different „outputs“ (yellow, blue, green) with only two molecules (NIP, FluA) building up the „input-pattern“ on the Origami-DNA-surface. <br><br> |
<h2>'''Subprojects:'''</h2> | <h2>'''Subprojects:'''</h2> | ||
[[Team:Freiburg/Modeling|Modeling]]<br> | [[Team:Freiburg/Modeling|Modeling]]<br> | ||
Revision as of 20:46, 28 October 2008
|
Project Report |
_project report
Introduction:This year´s main project is the attempt to create an "artificial receptor-system", featuring extra- and intracellular modules as well as suitable transmembrane regions.
The intracellular domaine of our receptor-device is build by halves of split reporter-proteins that can reassemble and will then produce readable output, e. g. fluorescence.
Each one of these protein-halves is connected to its extracellular domaine by a single-span transmembrane-helix.
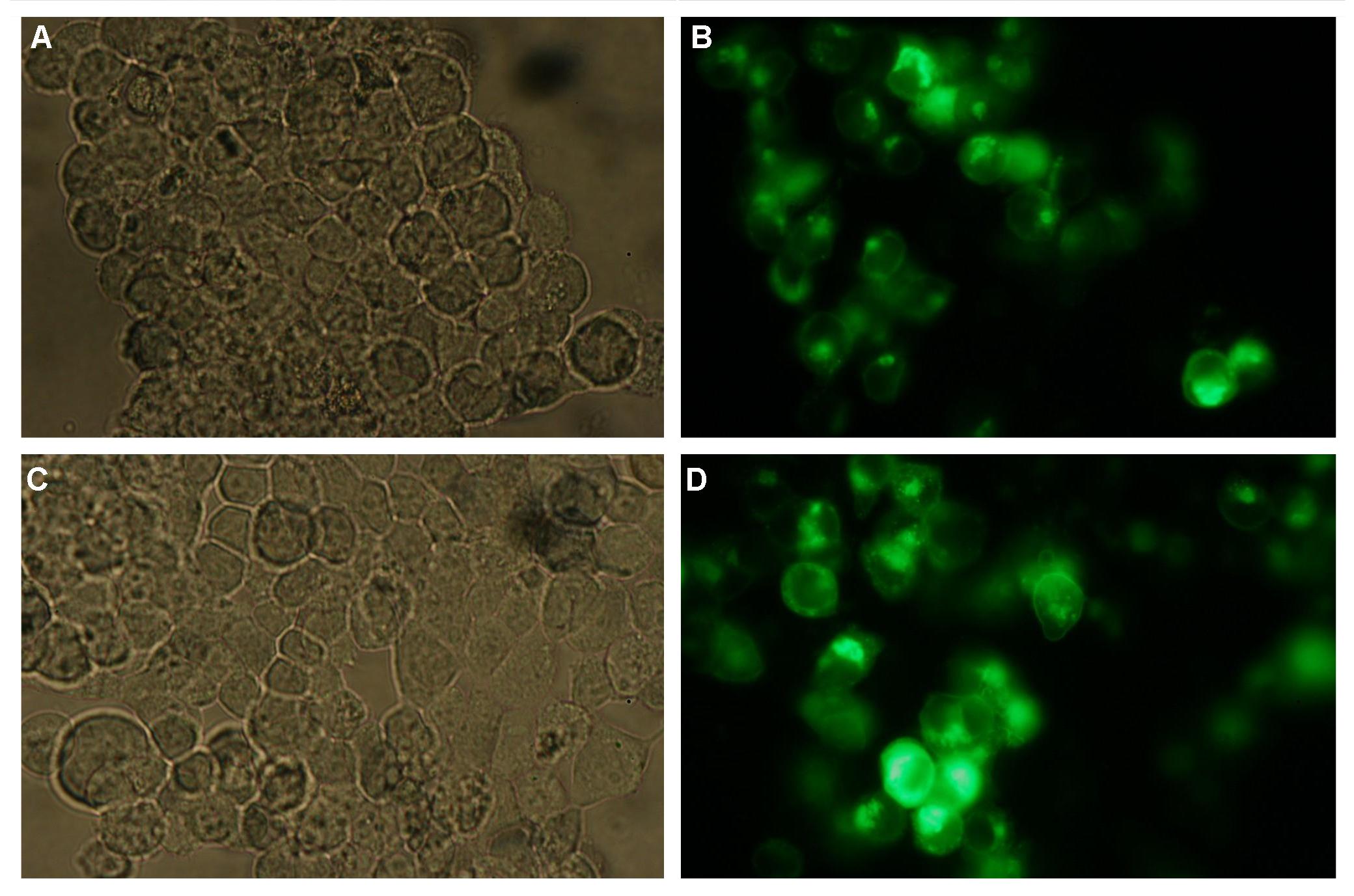
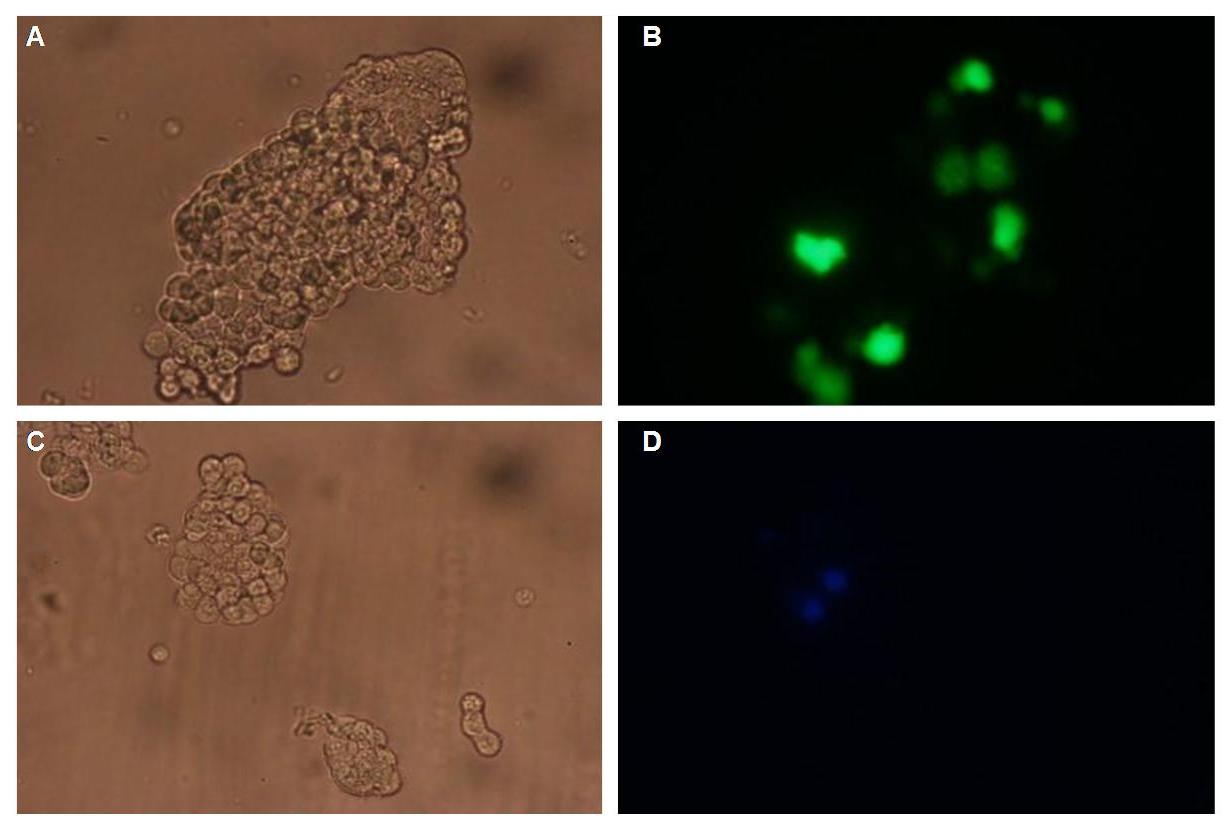
The extracellular or detecting domaine consists of a protein or peptide with the ability to bind a certain molecule. Subprojects:Modeling Results:All of our constructs (see "Parts") were cloned successfully using our extended pre- and suffix. Transfection of 293T-cells has also been shown to work for most of our fusion proteins; so far, we could even show that at least our constructs with lipocalin FluA as extracellular domaine are integrated into the membrane (Fig.1, details see Transfection). Discussion:Literature:Split-fluorophores: |
 "
"