Team:Chiba/Calendar-Home/10 September 2008
From 2008.igem.org
(Difference between revisions)
(→Laboratory work) |
(→Team:Communication) |
||
| (5 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
===Team:Communication=== | ===Team:Communication=== | ||
| - | (9/9)--> | + | (9/9)-->'''Colony Count''' |
| - | + | #LuxI([http://partsregistry.org/Part:BBa_K084012 BBa_K084012]) | |
| - | #LuxI | + | #LuxI+LVA([http://partsregistry.org/Part:BBa_K084014 BBa_K084014]) |
| - | #LuxI | + | #LasI([http://partsregistry.org/Part:BBa_K084007 BBa_K084007]) |
| - | # | + | #Background([http://partsregistry.org/Part:BBa_R0010 R0010]) |
| - | #Background(R0010) | + | #Background([http://partsregistry.org/Part:BBa_C0261 BBa_C0261]) |
| - | #Background( | + | |
Pick colony | Pick colony | ||
'''[[Team:Chiba/protocol/PCR|Colony-PCR]]''' | '''[[Team:Chiba/protocol/PCR|Colony-PCR]]''' | ||
| - | ::Colony PCR of 10 colonies from ligation plate(9/9-(2)) and 3 colonies from ligation plate(9/9-(3)) one from control plate([http://partsregistry.org/Part: | + | ::Colony PCR of 10 colonies from ligation plate(9/9-(2)) and 3 colonies from ligation plate(9/9-(3)) one from control plate([http://partsregistry.org/Part:BBa_F2620 BBa_F2620](2007)). |
:<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | :<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| Line 26: | Line 25: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dNTP mix( | + | <td>dNTP mix(μL)</td> |
<td>5</td> | <td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Foward Primer( | + | <td>Foward Primer(μL)</td> |
<td>0.3</td> | <td>0.3</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Reverse Primer( | + | <td>Reverse Primer(μL)</td> |
<td>0.3</td> | <td>0.3</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>taq DNA polymerase( | + | <td>taq DNA polymerase(μL)</td> |
<td>0.5</td> | <td>0.5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Thermopol Buffer( | + | <td>Thermopol Buffer(μL)</td> |
<td>3</td> | <td>3</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Nuclease free water( | + | <td>Nuclease free water(μL)</td> |
<td>20</td> | <td>20</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL( | + | <td>TOTAL(μL)</td> |
<td>30</td> | <td>30</td> | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | + | ||
| - | : | + | :95°C,5min -> ( 95°C,1min -> 52°C,1min -> 72°C,1min )・・・25cycles -> 72°C,10min -> 6°C |
| - | + | ||
-->'''[[Team:Chiba/protocol/gelcheck|Gel Check]]''' | -->'''[[Team:Chiba/protocol/gelcheck|Gel Check]]''' | ||
| + | {|align="justify" | ||
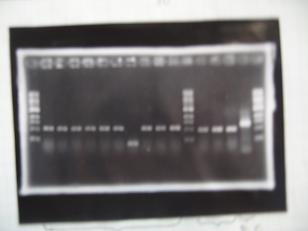
| + | |[[Image:Chiba-0910.JPG]] | ||
| + | |||
| + | :<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample DNA</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Loading Dye</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O</td> | ||
| + | <td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL</td> | ||
| + | <td>6μL</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | | | ||
| + | :From left; | ||
| + | ::Marker | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> Bad | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084007 BBa_K084007] -> OK | ||
| + | ::Marker | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084014 BBa_K084014] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084014 BBa_K084014] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_K084014 BBa_K084014] -> OK | ||
| + | ::#[http://partsregistry.org/Part:BBa_F2620 BBa_F2620] ---control -> OK | ||
| + | ::Marker | ||
| + | |} | ||
| + | |||
--->(28/8)'''[[Team:Chiba/protocol/DNA Purification/sigma|Miniprep]]''' | --->(28/8)'''[[Team:Chiba/protocol/DNA Purification/sigma|Miniprep]]''' | ||
| - | eluted with | + | eluted with 50μL of TE buffer. |
===Team:Output=== | ===Team:Output=== | ||
Latest revision as of 23:10, 29 October 2008
9 September 2008 <|> 11 September 2008
Contents |
Laboratory work
Team:Input
no work
Team:Communication
(9/9)-->Colony Count
- LuxI([http://partsregistry.org/Part:BBa_K084012 BBa_K084012])
- LuxI+LVA([http://partsregistry.org/Part:BBa_K084014 BBa_K084014])
- LasI([http://partsregistry.org/Part:BBa_K084007 BBa_K084007])
- Background([http://partsregistry.org/Part:BBa_R0010 R0010])
- Background([http://partsregistry.org/Part:BBa_C0261 BBa_C0261])
Pick colony
- Colony PCR of 10 colonies from ligation plate(9/9-(2)) and 3 colonies from ligation plate(9/9-(3)) one from control plate([http://partsregistry.org/Part:BBa_F2620 BBa_F2620](2007)).
DNA Template(μL) 1 dNTP mix(μL) 5 Foward Primer(μL) 0.3 Reverse Primer(μL) 0.3 taq DNA polymerase(μL) 0.5 Thermopol Buffer(μL) 3 Nuclease free water(μL) 20 TOTAL(μL) 30
- 95°C,5min -> ( 95°C,1min -> 52°C,1min -> 72°C,1min )・・・25cycles -> 72°C,10min -> 6°C
-->Gel Check
--->(28/8)Miniprepeluted with 50μL of TE buffer.
Team:Output
-->Gel check
-->Mini prep
 "
"