Team:Harvard/Parts/Tempsenseci
From 2008.igem.org
(→BBa_K098988) |
|||
| (34 intermediate revisions not shown) | |||
| Line 55: | Line 55: | ||
{|align="justify" style="background-color:#FFFFFF;text-indent: 15pt;text-align:justify" cellpadding="50" width="90%" | {|align="justify" style="background-color:#FFFFFF;text-indent: 15pt;text-align:justify" cellpadding="50" width="90%" | ||
| | | | ||
| - | = | + | =Thermoinducible cI System= |
| - | + | This system uses a a temperature sensitive variant of cI lambda to regulate the lambda promoter. | |
| + | The thermoinducible cI lambda system uses cI857 (a mutant form of cI from [http://www.addgene.org/pgvec1?f=c&vectorid=5079&cmd=genvecmap&dim=800&format=html&mtime=1188314819 pGW7] purchased from [http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx#40554 ATCC]) to regulate expression of genes under the control of the lambda promoter. The cI857 repressor is repressed by thermal denaturation. Activity of cI857 begins to decrease around 30 ºC and is fully denatured by around 42 ºC (Leipold et al., 1994). Thus transcription of the gene under the control of the lambda promoter can be induced by increasing the temperature from 30 ºC to 37 ºC-40 ºC. | ||
| - | + | We had previously tried to use the thermoinducible lac system in the Registry ([http://partsregistry.org/Part:BBa_J06912 BBa_J06912] and [http://partsregistry.org/Part:BBa_J06911 BBa_J06911]). However, induction tests, a [https://2008.igem.org/Team:Harvard/Dailybook/Week6/Chemical_and_Light#Western_Blot Western blot], and sequencing confirmed that these parts are not functional. We thus focused our efforts on creating a thermosensitive cI system. | |
| - | + | ||
| - | + | ||
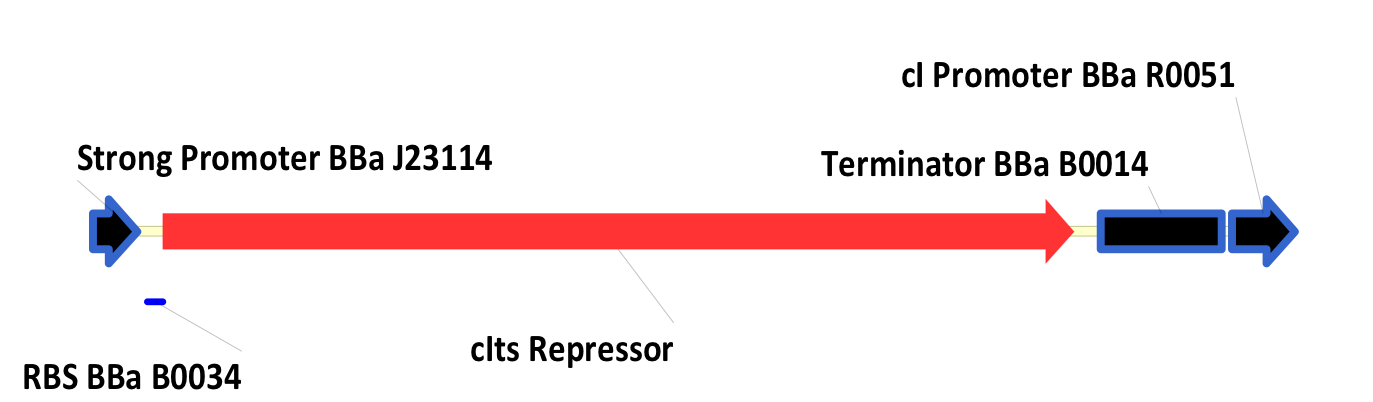
| + | ===BBa_K098995=== | ||
| + | This is a thermosensitive cI inducible system driven by a strong promoter. | ||
| + | <div style="text-indent:0pt;color:black">[[Image:122.png|thumb|650px|center|BBa_K098995]]</div> | ||
| + | |||
| + | [http://partsregistry.org/Part:BBa_K098993 BBa_K098993] is similar, with the strong promoter at the beginning of the part replaced by a weak promoter. | ||
| + | |||
| + | ==Induction Test for Thermosensitive cI Systems with GFP Reporters== | ||
| + | An induction test was designed to test the inducibility of the heat sensitive cI systems. Two constructs were made: | ||
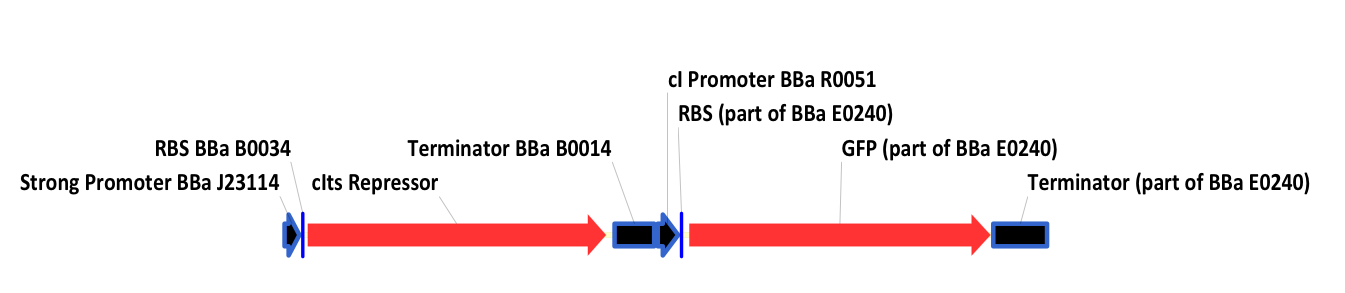
| + | ===BBa_K098988=== | ||
| + | This is a thermosensitive cI inducible system driven by a strong promoter and with a GFP indicator. | ||
| + | <div style="text-indent:0pt;color:black">[[Image:124.png|thumb|650px|center|BBa_K098988]]</div> | ||
| + | |||
| + | [http://partsregistry.org/Part:BBa_K098987 BBa_K098987] is equivalent, except that a weak promoter replaces the strong promoter at the beginning of the BBa_K099988. | ||
| + | |||
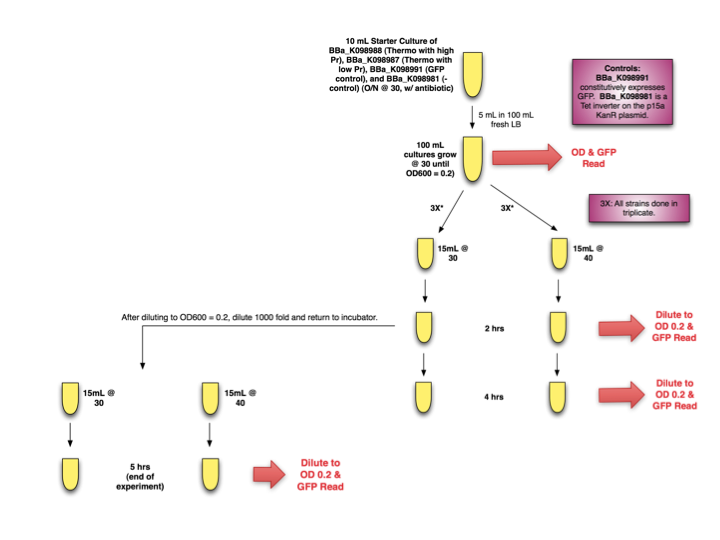
| + | ===Experimental Design=== | ||
| + | <div style="text-indent:0pt;color:black">[[Image:Thermo.png|thumb|150px|Thermoinduction Experimental Design]]</div> | ||
| + | Starter cultures of E. coli with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K098988| BBa_K098988] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K098987| BBa_K098987] were grown overnight. They were then diluted and grown to OD 0.2 before separation into induced (40 ºC) and uninduced cultures (30 ºC). OD and GFP readings were taken at time 0, 2, and 4 hours. Additionally, after diluting T=2hrs samples to OD 0.2 for accurate GFP measurements, samples were further diluted 1000x, induced (or not induced) again, and placed back in their respective incubators until the end of the experiment, when OD and GFP readings were taken. | ||
| + | |||
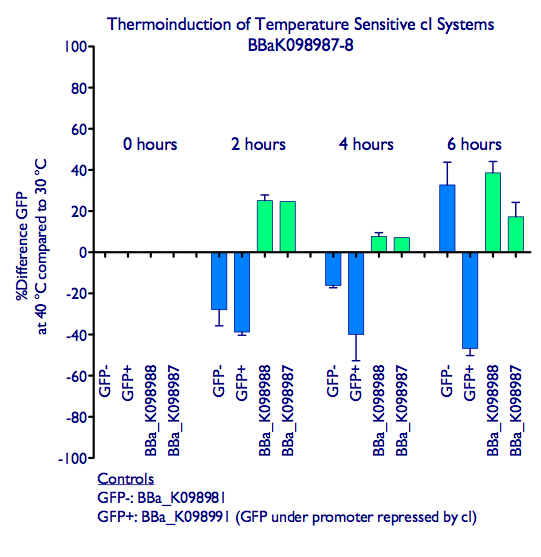
| + | ===Results=== | ||
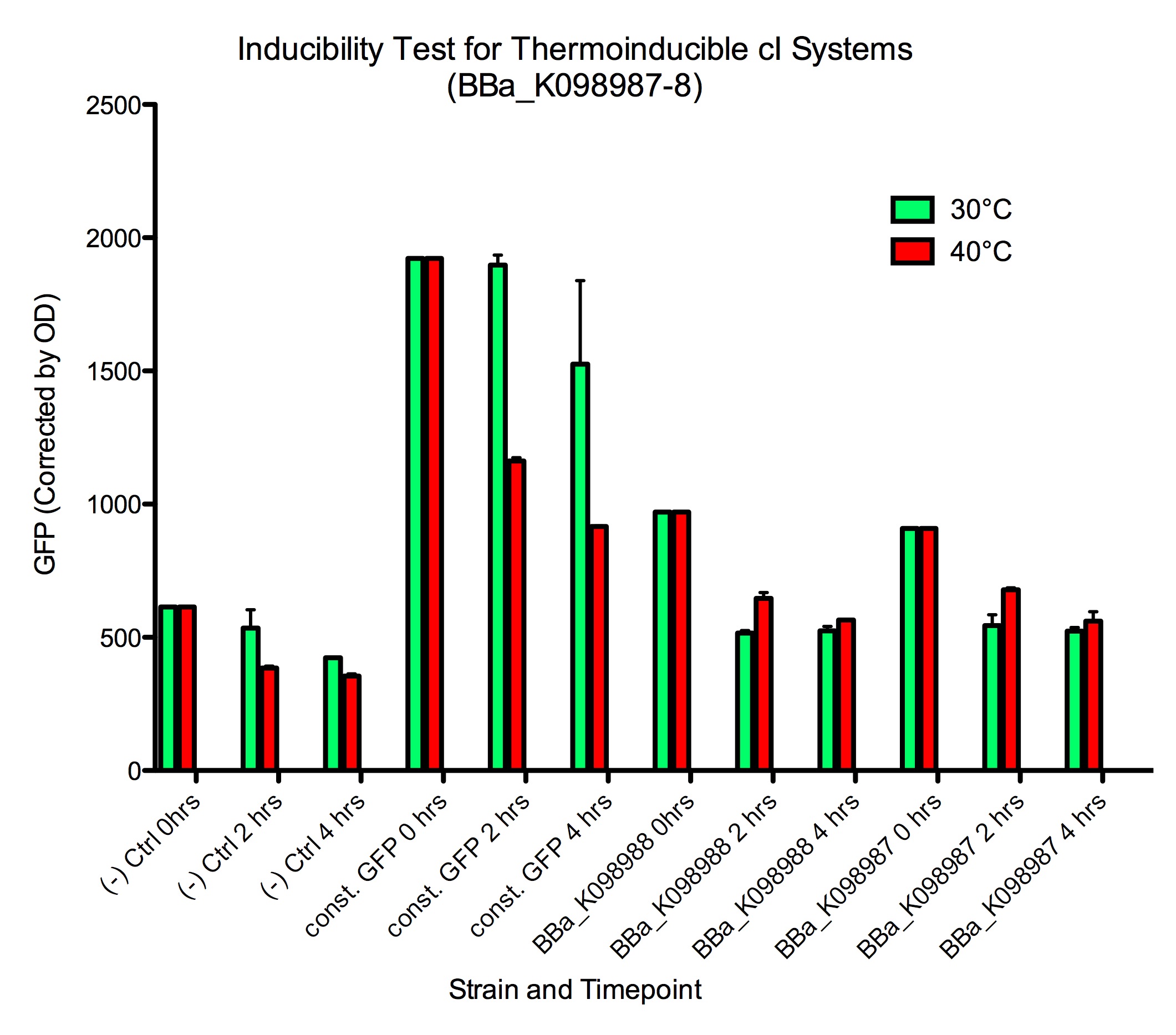
| + | Induction of GFP expression was observed at both 2 and 4 hours after moving samples to 40 ºC. While levels of GFP expression in the GFP+ control ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K098991| BBa_K098991]) went down in the samples at 40 ºC, levels in the inducible systems increased slightly. Note that the ''absolute levels'' of GFP expression do not increase much relative to 30 ºC (see our raw data at at the bottom of the page), but the increase in GFP expression is significantly different behavior from the decrease observed in the GFP+ control. We hypothesize that elevated temperature affects the GFP expression (e.g. by disrupting protein folding), and that even the small increase in GFP expression with our systems indicates effective induction. However, this does suggest that such a system is not optimal for inducing the expressing of heat-sensitive proteins. | ||
| + | |||
| + | <div style="text-indent:0pt;color:black">[[Image:CIts.png|780px|thumb|center|A comparison of GFP expression following thermoinduction of cells harboring BBa_K098987, BBa_K098988, a constitutive GFP generator (GFP+ control), and a plasmid not encoding GFP (GFP- control). The GFP readings were normalized by culture OD.]]</div> | ||
| + | |||
| + | |||
| + | Should it help you, we also have the raw values graphed below. Note that a slight decrease in baseline fluorescence was also observed in negative control GFP- cells ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K098981| BBa_K098981]). | ||
| + | |||
| + | <div style="text-indent:0pt;color:black">[[Image:Thermo_all.jpg|720px|thumb|center|Thermoinduction, raw data]]</div> | ||
| + | |||
| + | Results from the 1000x dilutions were inconclusive because the E. coli grows much slower at 30 ºC than at 40 ºC, so by the end of the experiment, there was a vast difference in cell concentration between the two sets of samples. | ||
| + | |||
| + | |} | ||
|} | |} | ||
<br><br> | <br><br> | ||
<!--- end body ---> | <!--- end body ---> | ||
|} | |} | ||
Latest revision as of 04:58, 30 October 2008
|
|
 "
"