Team:Heidelberg/Notebook/Sensing Group/Notebook/6thweek
From 2008.igem.org
(Difference between revisions)
Chenchenzhu (Talk | contribs) (→Monday, 09/08/2008) |
|||
| Line 466: | Line 466: | ||
== Monday, 09/08/2008 == | == Monday, 09/08/2008 == | ||
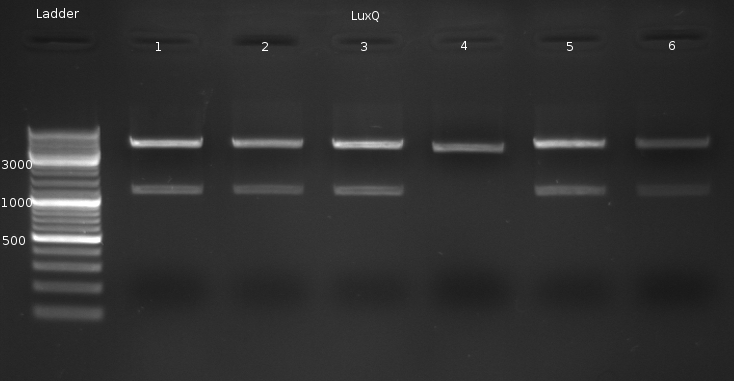
| - | * | + | * Culture of 5 colonies from LuxQ Transformation and 1 from F1 transformation |
| - | * Miniprep of | + | * Miniprep of Culture from previous Transformation (done by Chenchen). Incubated for two days @ RT and about then 6 h @ 37 °C |
| - | * Digestion of the Miniprep products with | + | * Digestion of the Miniprep products with XbaI (NEBuffer 2 + BSA, 1 ul enzyme @ 37 °C about 40min) |
[[Image:HD 080908-LuxQ Colonies-Digestion xbaI.png|center|thumb|500px|Digestion of LuxQ with xbaI yielded expected bands (4736 + 1204 bp) for all samples, except for no. 4]] | [[Image:HD 080908-LuxQ Colonies-Digestion xbaI.png|center|thumb|500px|Digestion of LuxQ with xbaI yielded expected bands (4736 + 1204 bp) for all samples, except for no. 4]] | ||
| - | Sequencing @ GATC | + | Sequencing @ GATC verified correct sequence for LuxQ no. 1 |
* PCR for LuxQ and LuxP with Phusion (25 µl Mastermix, 0.5 µl each Primer, 0(1) µl DMSO, 24(23) µl H<sub>2</sub>0, 0.5 µl Template) | * PCR for LuxQ and LuxP with Phusion (25 µl Mastermix, 0.5 µl each Primer, 0(1) µl DMSO, 24(23) µl H<sub>2</sub>0, 0.5 µl Template) | ||
* PCR Programme: 5 min @ 98 °C || 10 s @ 98 °C | 30 s @ 55 °C | 30 s @ 72 °C || 5 min @ 72 °C | 4 °C | * PCR Programme: 5 min @ 98 °C || 10 s @ 98 °C | 30 s @ 55 °C | 30 s @ 72 °C || 5 min @ 72 °C | 4 °C | ||
Revision as of 18:21, 28 October 2008


Contents |
Monday, 09/08/2008
- Culture of 5 colonies from LuxQ Transformation and 1 from F1 transformation
- Miniprep of Culture from previous Transformation (done by Chenchen). Incubated for two days @ RT and about then 6 h @ 37 °C
- Digestion of the Miniprep products with XbaI (NEBuffer 2 + BSA, 1 ul enzyme @ 37 °C about 40min)
Sequencing @ GATC verified correct sequence for LuxQ no. 1
- PCR for LuxQ and LuxP with Phusion (25 µl Mastermix, 0.5 µl each Primer, 0(1) µl DMSO, 24(23) µl H20, 0.5 µl Template)
- PCR Programme: 5 min @ 98 °C || 10 s @ 98 °C | 30 s @ 55 °C | 30 s @ 72 °C || 5 min @ 72 °C | 4 °C
Tuesdsay, 09/09/2008
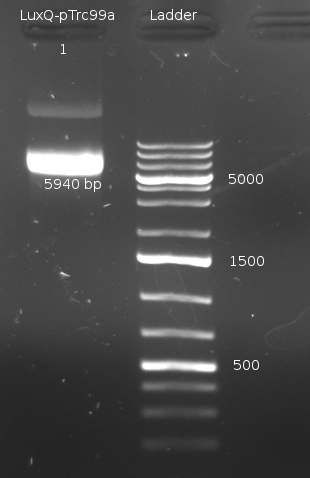
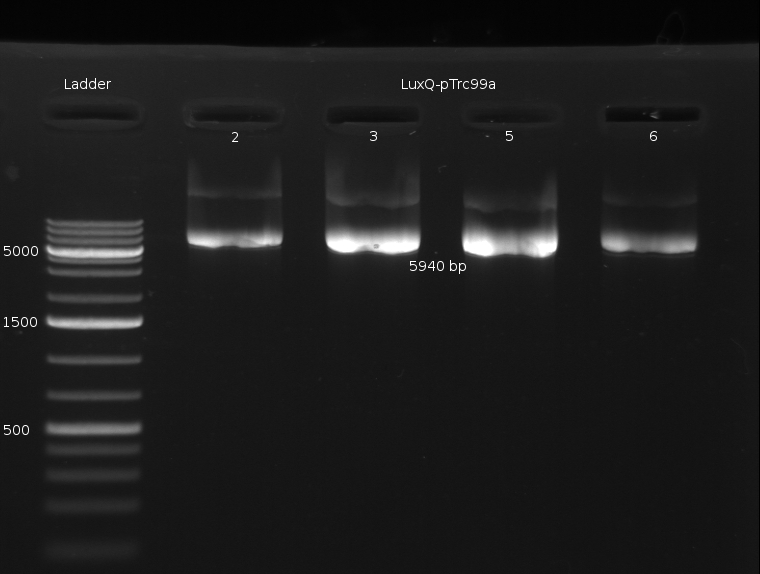
- Miniprep of LuxQ colonies no. 1,2,3,5,6
- PCR for LuxQ fragment 1 and 2, Tar fragment 1 and 2 for Phusion receptor with Phusion-Polymerase
- PCR Programme: 30 s @ 98 °C || 10 s @ 98 °C | 30 s @ 55 °C |30 s @ 72 °C || 5 min @ 72 °C | 4 °C (30 cycles)
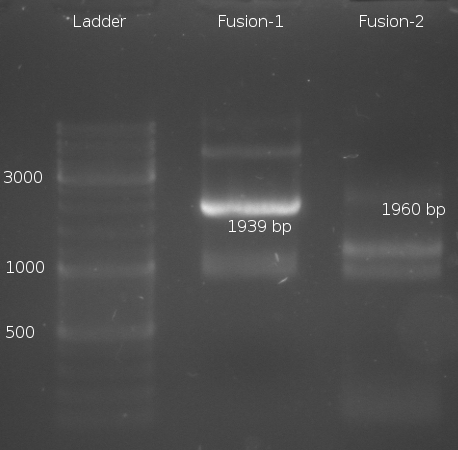
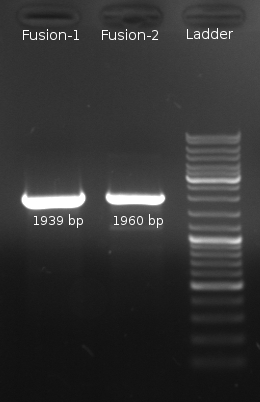
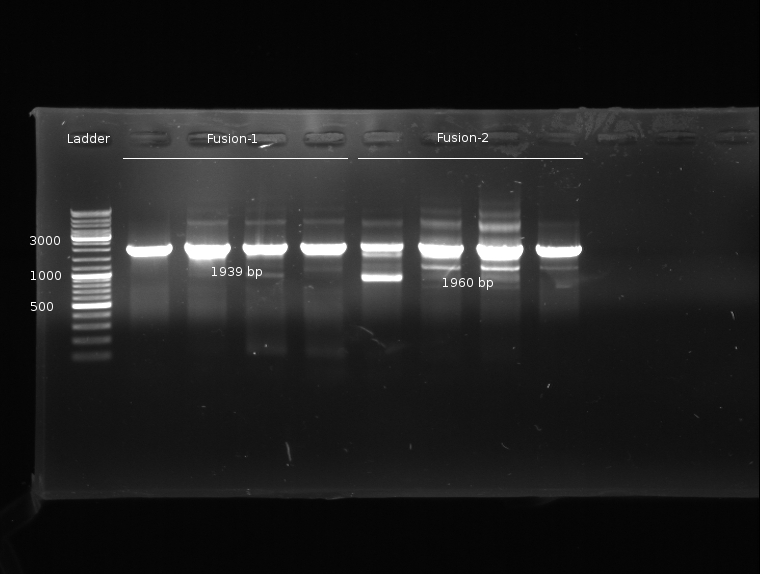
- Fusion-PCR for Fusion-1 and Fusion-2 constructs
- O/N Culture of 10 LuxP in pDK48 colonies
Wednesday, 09/10/2008
LuxP cloning
- Miniprep of O/N LuxP cultures and digestion with NcoI (NEBuffer 2, 2h @ 37 °C)
Sequencing @ GATC: LuxP1 correct
Fusion chimeras
- PCR for LuxQ 1+2, Tar 1+2 and Gel Extraction to get rid of first primers
- Fusion-PCR of LuxQ1+Tar1 und LuxQ2+Tar2 with Phusion and 55 °C annealing --> No product
- PCR of LuxQ fragment 1b, LuxQ fragment 1c, LuxQ fragment 2b, LuxQ fragment 2c, Tar fragment 1b, Tar fragment 1c, Tar fragment 2b, Tar fragment 2c with Phusion and 55 °C annealing. Products were purified via Gelextraction to get rid of first primers
- Two Fusion PCR (30 s @ 98 °C || 10 s @ 98 °C | 30 s @ 55 °C |30 s @ 72 °C || 5 min @ 72 °C | 4 °C (30 cycles)) with PCR purification.
- Gel Extraction of LuxQ 1+2, Tar 1+2
Thursday, 09/11/2008
Fusion chimeras
- PCR Purification of previous Fusion PCR
- Digestion with NcoI/NdeI (NEBuffer4) and NcoI/KpnI (NEBuffer1 + BSA). 1 h @ 37 °C
- Gelextraction, eluted in 30 µl
- Ligation (Insert:Vector 1:1) and transformation into DH5a competent cells
Friday, 09/12/2008
nothing to report
 "
"