Team:KULeuven/Model/Inverter
From 2008.igem.org
(Difference between revisions)
m (→Matlab (SBML file)) |
(→Simulations) |
||
| Line 179: | Line 179: | ||
=== Simulations === | === Simulations === | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Time span | ||
| + | ! Input (TetR) | ||
| + | ! Results | ||
| + | |- | ||
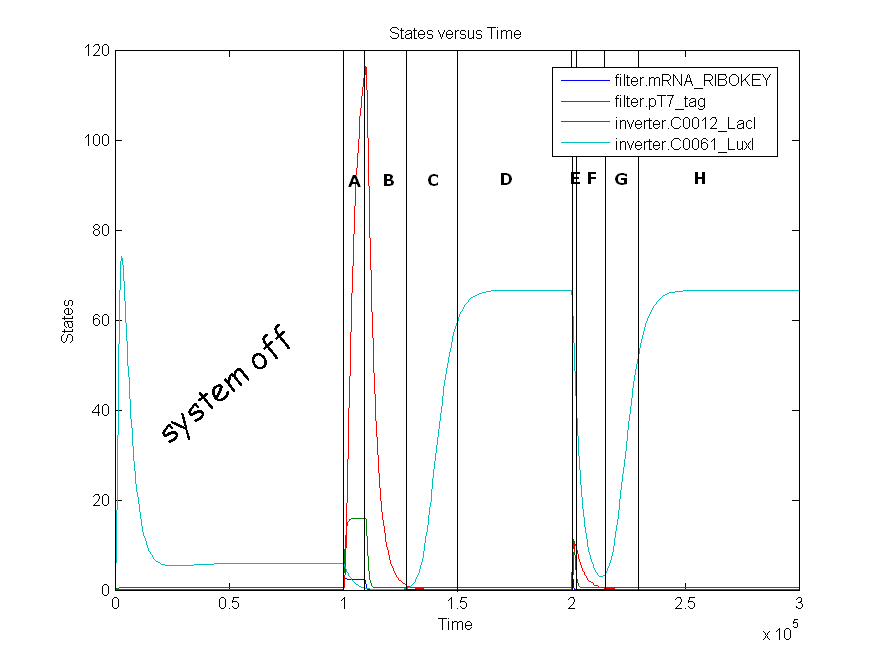
| + | | A | ||
| + | | 0.0125 | ||
| + | | The amount LacI increases from state zero to state one because both mRNA_RIBOKEY and pT7_tag are present. This results in a repression of LuxI which decreases to zero. | ||
| + | |- | ||
| + | | B | ||
| + | | 5E-5 | ||
| + | | | ||
| + | |- | ||
| + | | C | ||
| + | | 5E-5 | ||
| + | | | ||
| + | |- | ||
| + | | D | ||
| + | | 5E-5 | ||
| + | | | ||
| + | |- | ||
| + | | E | ||
| + | | 0.0125 | ||
| + | | | ||
| + | |- | ||
| + | | F | ||
| + | | 5E-5 | ||
| + | | | ||
| + | |- | ||
| + | | G | ||
| + | | 5E-5 | ||
| + | | | ||
| + | |- | ||
| + | | H | ||
| + | | 5E-5 | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
Remark: simulation is not from latest version. | Remark: simulation is not from latest version. | ||
Revision as of 13:12, 8 September 2008
dock/undock dropdown
Contents |
Invertimer
Position in the system
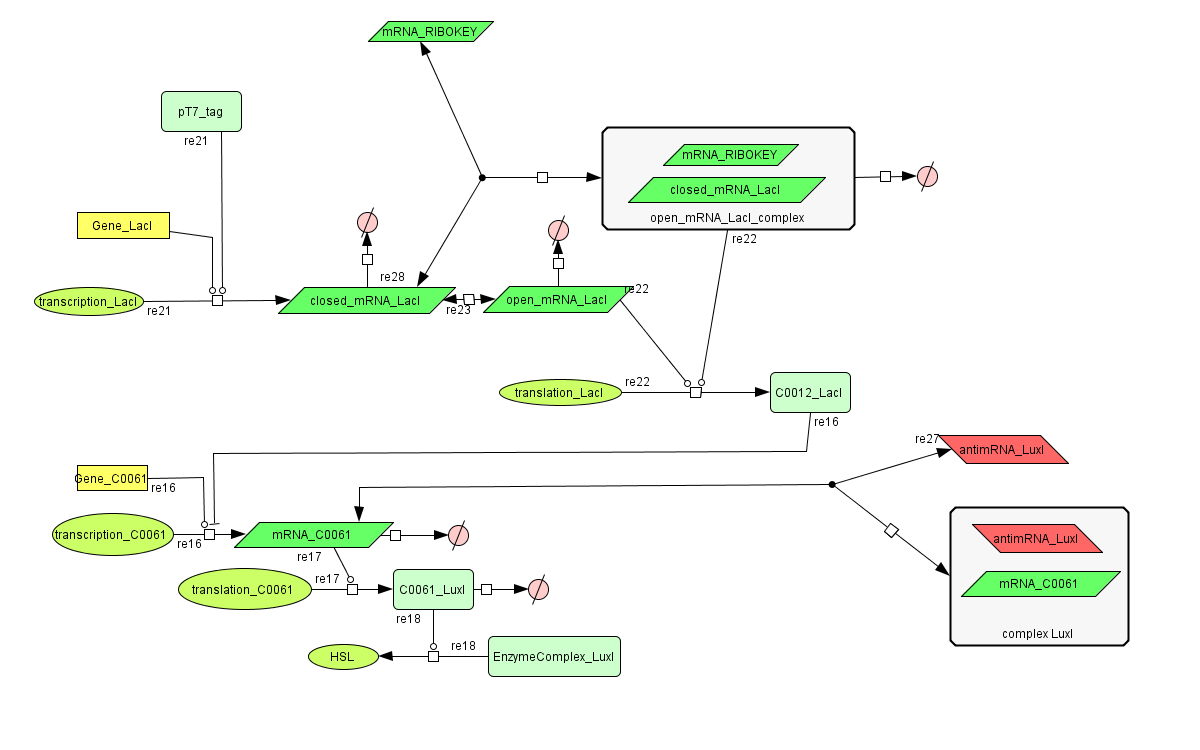
The invertimer subsystem receives its input from the filter, T7. The invertimer's function is to produce HSL when no input is present, so a low T7 input gives rise to a high HSL output and vice versa. The production of HSL means that the cell will start a timer that eventually will be used in the celldeath-subsystem to produce ccdB. In this way the cell will die off if no desease remains present.
Describing the system
see also: Project:Invertimer
ODE's
Parameters
| Name | Value | Comments | Reference |
|---|---|---|---|
| Degradation Rates | |||
| dLuxI | dLVA = 2.814E-4 s-1 | LVA-tag reduces lifetime to 40 minutes | [http://parts.mit.edu/igem07/index.php/ETHZ/Parameters link] [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=106306 link] |
| dRNA_LuxI | 0.0025 s-1 | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | |
| dLuxI_antimRNA | 0.0045303737 s-1 | estimate: because this RNA isn't translated, it degrades faster | |
| dLacI | dLVA = 2.814E-4 s-1 | LVA-tag reduces lifetime to 40 minutes | [http://parts.mit.edu/igem07/index.php/ETHZ/Parameters link] [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=106306 link] |
| dclosed mRNA LacI | 0.0046209812 s-1 | estimate: because this mRNA isn't translated, it degrades faster | |
| dopen mRNA LacI | 0.0023104906 s-1 | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | |
| dopen mRNA LacI complex | 0.0023104906 s-1 | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | |
| dHSL | 1.02E-6 s-1 | very stable in the medium, lifetime around 185h | [http://aem.asm.org/cgi/content/abstract/71/3/1291 link] |
| LuxI catalysis | |||
| kcat | 0.0166666667 s-1 | Estimated to be about 90% of Vmax in LB medium. | [http://www.pnas.org/content/93/18/9505 link] |
| T7 Transcription | |||
| KT7 | 421 | dissociation constant, recalculated to remove units | [http://www.jbc.org/cgi/content/full/279/5/3239 link] |
| kmax | 0.044 s-1 | maximal T7 transcription rate | [http://www.jbc.org/cgi/content/full/279/5/3239 link] |
| Key-Lock constants | |||
| Keq 1 | 0,015 [M] | between closed and open T7 mRNA, experimental | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| Keq 2 | 0.0212 [M] | between closed T7 mRNA and key unlocked mRNA complex, experimental | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kdis2 | 0.00416 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kcomplex2 | 0.00237 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kclosed | 500 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kopen | 7.5 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| LacI repression | |||
| KLacI | 1.0E-10 M-1 | Dissociation constant | |
| nLacI | 2.0 | Hill coefficient for LacI | [http://parts.mit.edu/igem07/index.php/ETHZ/Parameters link] |
| k_trans_LacI | 0.0025 s-1 | Estimated maximal transcription rate from R0011 | [http://partsregistry.org/Part:BBa_R0011 link] |
| Antisense LuxI | |||
| k_complex3 | 0.00237 s-1 | rate constant for formation of asRNA - LuxI mRNA duplex | |
| KmRNA_LuxI:antisense_mRNA | 4.22E14 | Complex of LuxI mRNA with antisense mRNA | |
| Translation Rates | |||
| ktransl LuxI | 0.167 s-1 | translation rate for B0032 RBS (0.3 relative efficiency) | [http://partsregistry.org/Part:BBa_B0032 link] |
| ktransl LacI | 0.167 s-1 | lock defined translation rate for LacI | |
Models
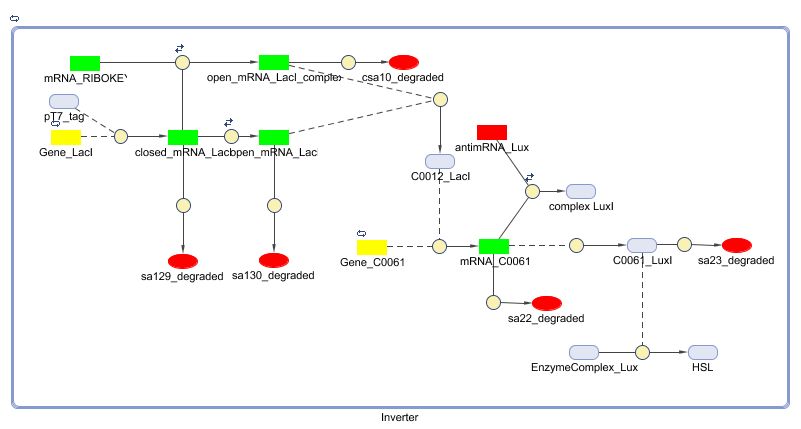
CellDesigner (SBML file)
Matlab (SBML file)
Simulations
| Time span | Input (TetR) | Results |
|---|---|---|
| A | 0.0125 | The amount LacI increases from state zero to state one because both mRNA_RIBOKEY and pT7_tag are present. This results in a repression of LuxI which decreases to zero. |
| B | 5E-5 | |
| C | 5E-5 | |
| D | 5E-5 | |
| E | 0.0125 | |
| F | 5E-5 | |
| G | 5E-5 | |
| H | 5E-5 |
Remark: simulation is not from latest version.
 "
"