Team:KULeuven/Model/Reset
From 2008.igem.org
(→Simulation) |
m (→Simulation) |
||
| Line 244: | Line 244: | ||
=== Simulation === | === Simulation === | ||
| - | During the first 50000 seconds, the input signal TetR is held constant at 5E-5. From 50000 till 80000 seconds, the input signal is at its maximum value: 0.0125. This results in an increase of lactonase. | + | During the first 50000 seconds, the input signal TetR is held constant at 5E-5. This results in a background signal of lactonase which will convert a small part of the HSL into hydroxy acid. From 50000 till 80000 seconds, the input signal is at its maximum value: 0.0125. This results in an increase of lactonase which converts almost every HSL molecule into the hydroxy acid. The timer is fully reset. After a delay of approximately 20000 seconds, the amount of HSL starts to increase back again: the clock ticks again. A shorter pulse of TetR (10000 seconds) only partially resets the timer: not all the HSL is converted into hydroxy acid. |
[[Image:Sim_lactonaseproduction_1.png|700px|center]] | [[Image:Sim_lactonaseproduction_1.png|700px|center]] | ||
Revision as of 08:49, 9 September 2008
Contents |
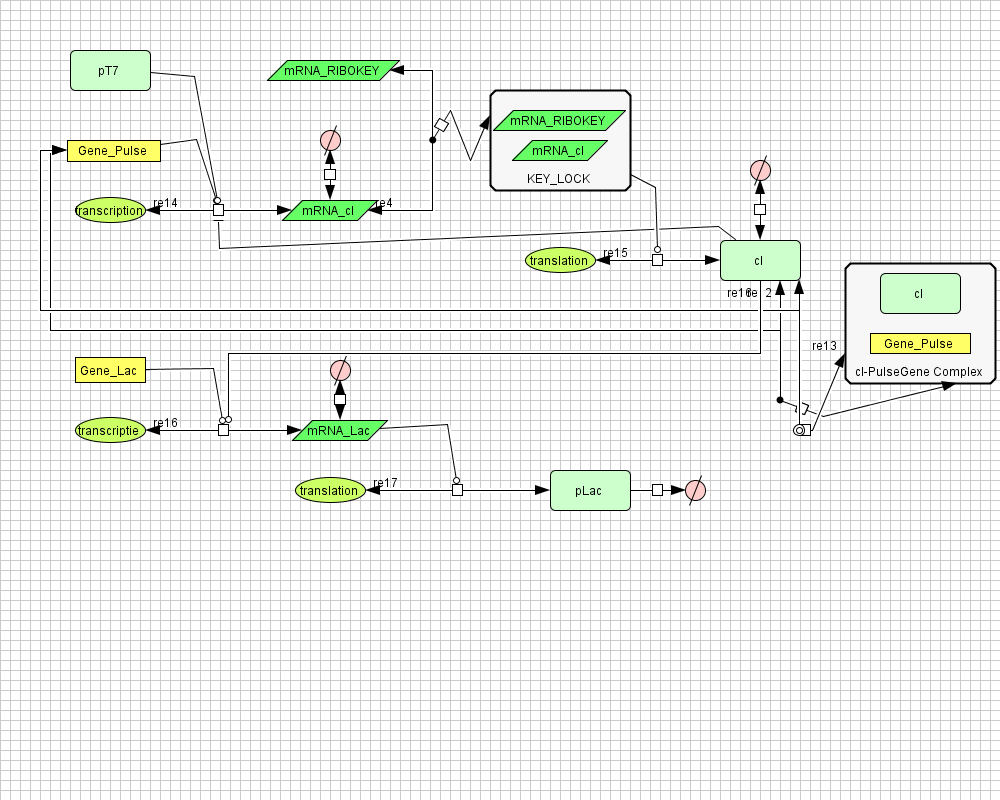
Pulse Generator
Position in the system
The Pulse Generator-subsystem is directly linked to the Filter.
When the filter indicates that the input is zero (there is no desease), the system will (ideally) produce no lactonase. As soon as the output of the filter is one, the subsystem will produce a pulse of lactonase which will be high enough to 'remove' all HSL present in the system and in that way reset the timer.
Describing the system
ODE's
![]() NOT AVAILABLE
NOT AVAILABLE
Parameters
| Name | Value | Comments | Reference |
|---|---|---|---|
| Degradation rates | |||
| dRNA_cI | 0.00462 s-1 | ||
| dcI | 7.0E-4 s-1 | [http://parts.mit.edu/igem07/index.php?title=ETHZ/Parameters link] | |
| dRNA_Lac | 0.00231 s-1 | ||
| dLac | 2.888E-4 s-1 | ||
| dRNA_Ribokey:cI | 0.00231 s-1 | ||
| Dissociation constants | |||
| KRibokey:cI | 0.00212 | kass/kdiss for the Ribokey cI complex | |
| KcI | 0.00337 | binding cI on cI-Promotor | [http://parts.mit.edu/igem07/index.php?title=ETHZ/Parameters link] |
| Transcription rates | |||
| kRNA_cI | 0.025 s-1 | maximal transcription rate RNA cI (no cI repressor present) | |
| kRNA_Lac | 0.025 s-1 | ||
| Translation rates | |||
| kcI | 0.167 s-1 | ||
| kLac | 0.167 s-1 | RBS is B0032 (efficiency 0.3) | [http://partsregistry.org/Part:BBa_B0032 link] |
| Hill cooperativity | |||
| ncI | 2.0 | [http://parts.mit.edu/igem07/index.php?title=ETHZ/Parameters link] | |
Models
CellDesigner (SBML file)
Matlab
Problem
The idea of a pulsgenerator as reset mechanism doesn't meet the black-box requirements for the following reasons:
- it takes too long before the proposed system generates a pulse-like event
- the pulse itself is too long
- a constant lactonase production sequence generates enough lactonase to reset the timer
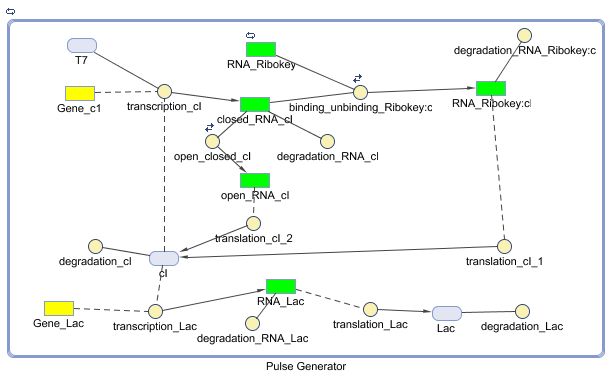
Constant Lactonase Production
Position in the system
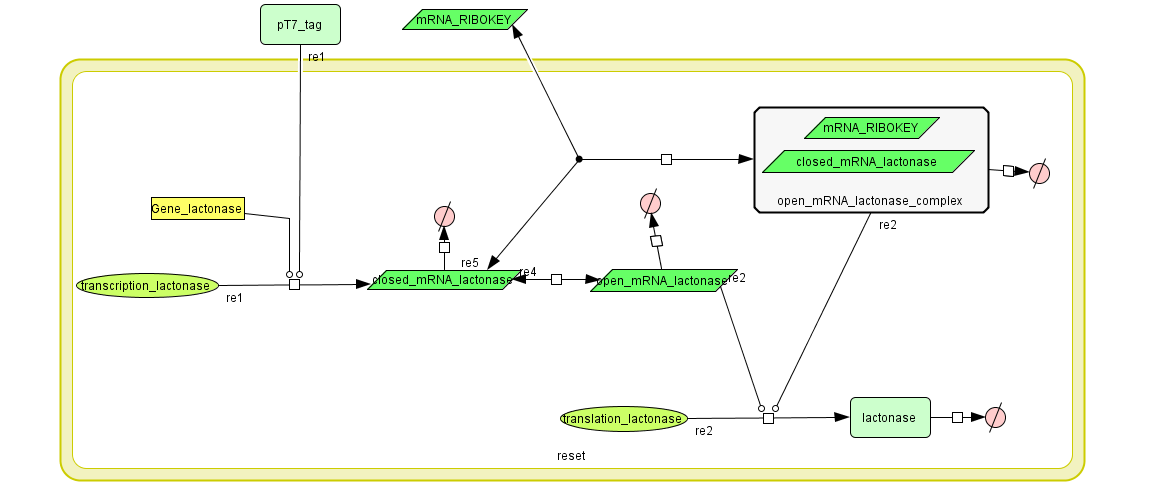
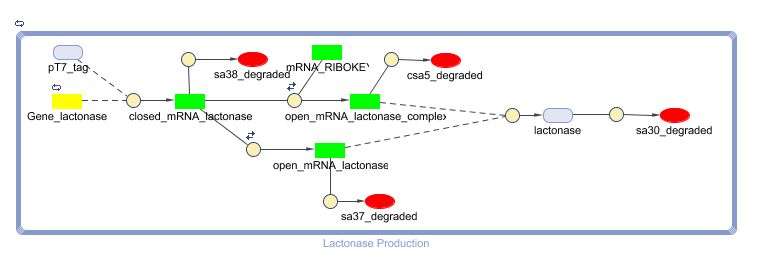
The Constant Lactonase Production-system is directly linked to the Filter.
When the filter indicates that the input is zero (there is no desease), the system will (ideally) produce no lactonase. As soon as the output of the filter is one, the system starts producing lactonase and remains doing this untill the light goes off again. In this way all the HSL-molecules that are present will be 'removed' and the timer is reset.
Describing the system
see also: Project:Reset
ODE's
Parameters
| Name | Value | Comments | Reference |
|---|---|---|---|
| Degradation rates | |||
| daiiA | dLVA = 2.814E-4 s-1 | LVA-tag reduces lifetime to 40 minutes | [3] |
| dclosed mRNA aiiA | 0.0046209812 s-1 | estimate: because this RNA isn't translated, it degrades faster | [2] |
| dopen mRNA aiiA | 0.0023104906 s-1 | [2] | |
| dmRNA aiiA complex | 0.0023104906 s-1 | [2] | |
| T7 Transcription | |||
| KT7 | 421 | dissociation constant, recalculated to remove units | [4] |
| kmax | 0.044 s-1 | maximal T7 transcription rate | [4] |
| Key-Lock constants | |||
| Keq 1 | 0,015 [M] | between closed and open Lactonase mRNA, modeled for competition, experimental | [1] |
| Keq 2 | 0.0212 [M] | between closed Lactonase mRNA and key unlocked mRNA complex, modeled for competition, experimental | [1] |
| kdis1 | 0.00416 s-1 | estimate: derived from experimental values | [1] |
| kcomplex1 | 0.00237 s-1 | estimate: derived from experimental values | [1] |
| kclosed | 500 s-1 | estimate: derived from experimental values | [1] |
| kopen | 7.5 s-1 | estimate: derived from experimental values | [1] |
| ktranslation | 0.167 s-1 | lock defined translation rate for Lactonase | |
Models
CellDesigner (SBML file)
Matlab (SBML file)
Simulation
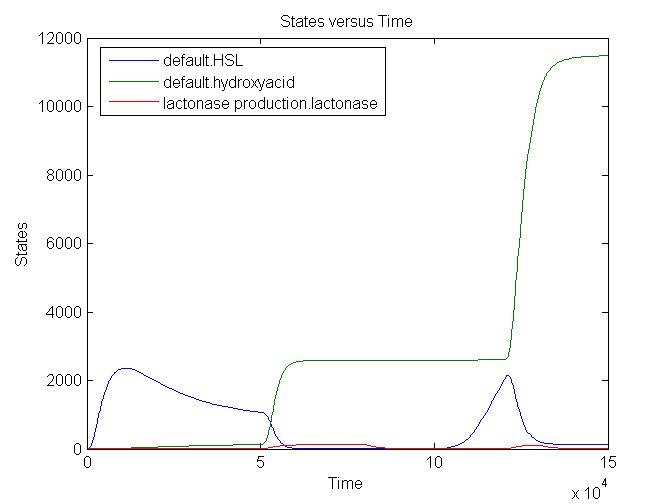
During the first 50000 seconds, the input signal TetR is held constant at 5E-5. This results in a background signal of lactonase which will convert a small part of the HSL into hydroxy acid. From 50000 till 80000 seconds, the input signal is at its maximum value: 0.0125. This results in an increase of lactonase which converts almost every HSL molecule into the hydroxy acid. The timer is fully reset. After a delay of approximately 20000 seconds, the amount of HSL starts to increase back again: the clock ticks again. A shorter pulse of TetR (10000 seconds) only partially resets the timer: not all the HSL is converted into hydroxy acid.
References
| [1] | “Berkeley2006-RiboregulatorsMain - IGEM”; http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain. |
| [2] | J.A. Bernstein et al., “Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays,” Proceedings of the National Academy of Sciences of the United States of America, vol. 99, Jul. 2002, pp. 9697–9702. |
| [3] | J.B. Andersen et al., “New Unstable Variants of Green Fluorescent Protein for Studies of Transient Gene Expression in Bacteria,” Applied and Environmental Microbiology, vol. 64, Jun. 1998, pp. 2240–2246. |
| [4] | G.M. Skinner et al., “Promoter Binding, Initiation, and Elongation By Bacteriophage T7 RNA Polymerase: A SINGLE-MOLECULE VIEW OF THE TRANSCRIPTION CYCLE,” J. Biol. Chem., vol. 279, Jan. 2004, pp. 3239-3244. |
 "
"