Team:KULeuven/Model/Reset
From 2008.igem.org
(→Parameters) |
m (→Parameters) |
||
| Line 163: | Line 163: | ||
! colspan="4" style="border-bottom: 1px solid #003E81;" | Degradation rates | ! colspan="4" style="border-bottom: 1px solid #003E81;" | Degradation rates | ||
|- | |- | ||
| - | | d<sub> | + | | d<sub>aiiA</sub> |
| | d<sub>LVA</sub> = 2.814E-4 s<sup>-1</sup> | | | d<sub>LVA</sub> = 2.814E-4 s<sup>-1</sup> | ||
| LVA-tag reduces lifetime to 40 minutes | | LVA-tag reduces lifetime to 40 minutes | ||
| [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=106306 link] | | [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=106306 link] | ||
|- | |- | ||
| - | | d<sub>closed mRNA | + | | d<sub>closed mRNA aiiA</sub> |
| 0.0046209812 s<sup>-1</sup> | | 0.0046209812 s<sup>-1</sup> | ||
| estimate: because this RNA isn't translated, it degrades faster | | estimate: because this RNA isn't translated, it degrades faster | ||
| [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | ||
|- | |- | ||
| - | | d<sub>open mRNA | + | | d<sub>open mRNA aiiA</sub> |
| 0.0023104906 s<sup>-1</sup> | | 0.0023104906 s<sup>-1</sup> | ||
| | | | ||
| [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | ||
|- | |- | ||
| - | | d<sub> | + | | d<sub>mRNA aiiA complex</sub> |
| 0.0023104906 s<sup>-1</sup> | | 0.0023104906 s<sup>-1</sup> | ||
| | | | ||
Revision as of 14:06, 28 August 2008
Contents |
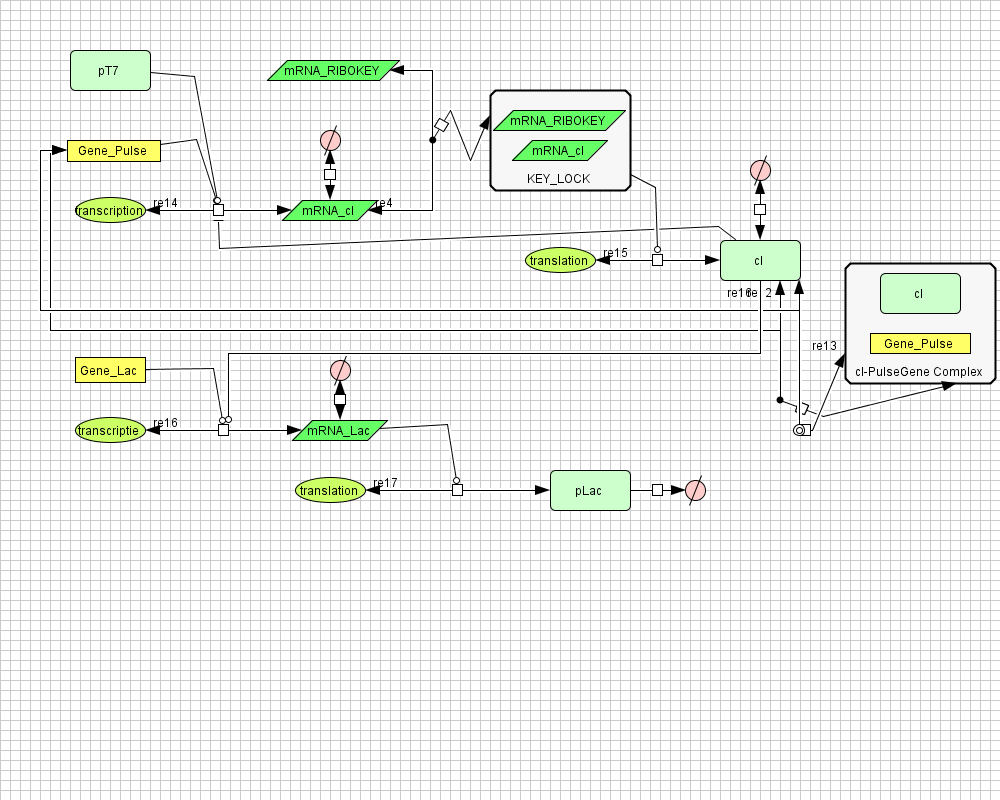
Pulse Generator
Position in the system
The Pulse Generator-subsystem is directly linked to the Filter.
When the filter indicates that the input is zero (there is no desease), the system will (ideally) produce no lactonase. As soon as the output of the filter is one, the subsystem will produce a pulse of lactonase which will be high enough to 'remove' all HSL present in the system and in that way reset the timer.
Describing the system
ODE's
![]() NOT AVAILABLE
NOT AVAILABLE
Parameters
| Name | Value | Comments | Reference |
|---|---|---|---|
| Degradation rates | |||
| dRNA_cI | 0.00462 s-1 | ||
| dcI | 7.0E-4 s-1 | [http://parts.mit.edu/igem07/index.php?title=ETHZ/Parameters link] | |
| dRNA_Lac | 0.00231 s-1 | ||
| dLac | 2.888E-4 s-1 | ||
| dRNA_Ribokey:cI | 0.00231 s-1 | ||
| Dissociation constants | |||
| KRibokey:cI | 0.00212 | kass/kdiss for the Ribokey cI complex | |
| KcI | 0.00337 | binding cI on cI-Promotor | [http://parts.mit.edu/igem07/index.php?title=ETHZ/Parameters link] |
| Transcription rates | |||
| kRNA_cI | 0.025 s-1 | maximal transcription rate RNA cI (no cI repressor present) | |
| kRNA_Lac | 0.025 s-1 | ||
| Translation rates | |||
| kcI | 0.167 s-1 | ||
| kLac | 0.167 s-1 | RBS is B0032 (efficiency 0.3) | [http://partsregistry.org/Part:BBa_B0032 link] |
| Hill cooperativity | |||
| ncI | 2.0 | [http://parts.mit.edu/igem07/index.php?title=ETHZ/Parameters link] | |
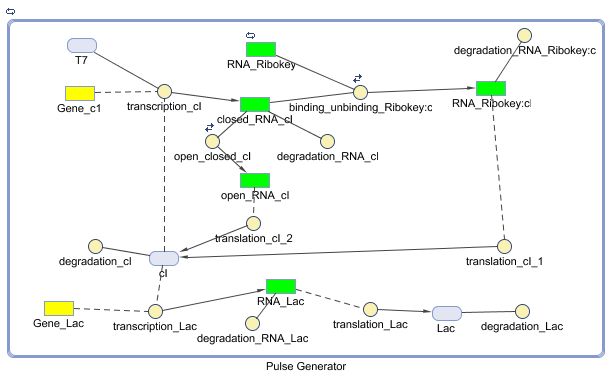
Models
CellDesigner (SBML file)
Matlab
Problem
The idea of a pulsgenerator as reset mechanism doesn't meet the black-box requirements for the following reasons:
- it takes too long before the proposed system generates a pulse-like event
- the pulse itself is too long
- a constant lactonase production sequence generates enough lactonase to reset the timer
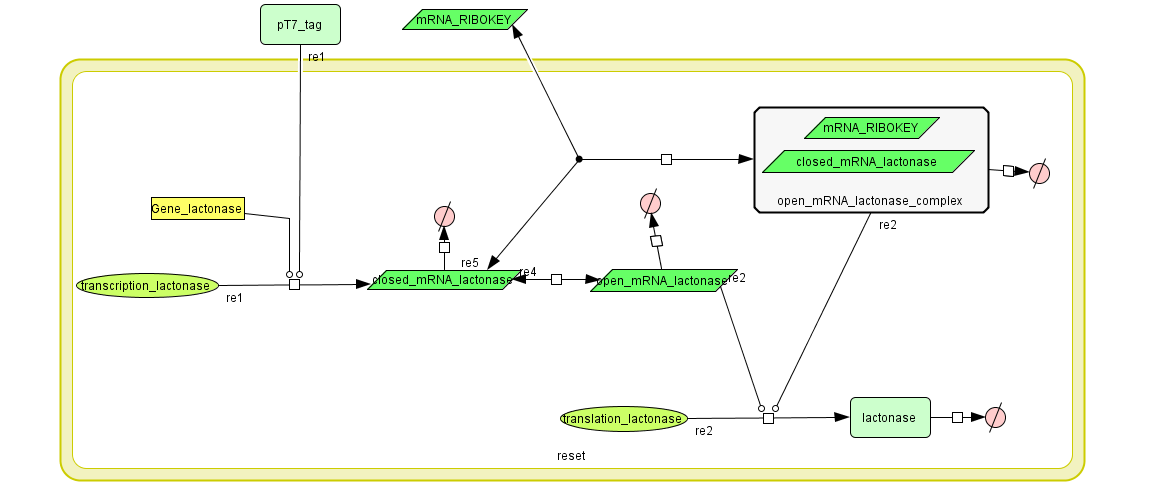
Constant Lactonase Production
Position in the system
The Constant Lactonase Production-system is directly linked to the Filter.
When the filter indicates that the input is zero (there is no desease), the system will (ideally) produce no lactonase. As soon as the output of the filter is one, the system starts producing lactonase and remains doing this untill the light goes off again. In this way all the HSL-molecules that are present will be 'removed' and the timer is reset.
Describing the system
see also: Project:Reset
ODE's
Parameters
| Name | Value | Comments | Reference |
|---|---|---|---|
| Degradation rates | |||
| daiiA | dLVA = 2.814E-4 s-1 | LVA-tag reduces lifetime to 40 minutes | [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=106306 link] |
| dclosed mRNA aiiA | 0.0046209812 s-1 | estimate: because this RNA isn't translated, it degrades faster | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] |
| dopen mRNA aiiA | 0.0023104906 s-1 | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | |
| dmRNA aiiA complex | 0.0023104906 s-1 | [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=124983&blobtype=pdf link] | |
| T7 Transcription | |||
| KT7 | 421 | dissociation constant, recalculated to remove units | [http://www.jbc.org/cgi/content/full/279/5/3239 link] |
| kmax | 0.044 s-1 | maximal T7 transcription rate | [http://www.jbc.org/cgi/content/full/279/5/3239 link] |
| Key-Lock constants | |||
| Keq 1 | 0,015 [M] | between closed and open Lactonase mRNA, experimental | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| Keq 2 | 0.0212 [M] | between closed Lactonase mRNA and key unlocked mRNA complex, experimental | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kdis1 | 100 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kcomplex1 | 57 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kclosed | 100 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| kopen | 1.5 s-1 | derived from experimental values | [http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain link] |
| ktranslation | 0.167 s-1 | lock defined translation rate for Lactonase | |
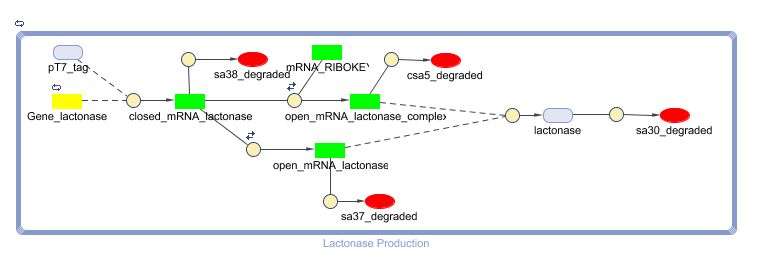
Models
CellDesigner (SBML file)
Matlab (SBML file)
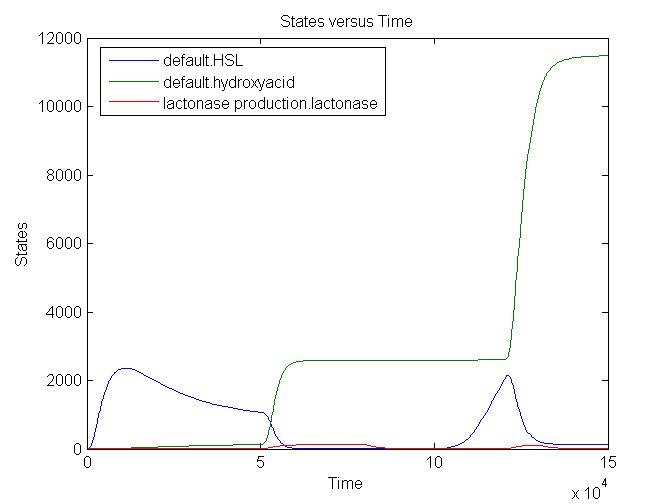
Simulation
Remark: up to date with latest version?
The number lactonase genes is held constant during the entire simulation. For the first 15.000 seconds the number of mRNA Ribokey is equal to 0.015 and the number of pT7 molecules to 0.4, then for 15000 s these numbers are set on 6 and 3 respectively (based on the results of the model of the filter) after which they are reduced back to 0.015 and 0.4.
We see that an increase in the number of mRNA Ribokey and pT7(due to an increase in light intensity) will lead to a much higher number of lactonase molecules.
 "
"