Team:Paris/August 15
From 2008.igem.org
(Difference between revisions)
(→Results) |
(→Results) |
||
| Line 56: | Line 56: | ||
==='''Results'''=== | ==='''Results'''=== | ||
| + | |||
| + | |||
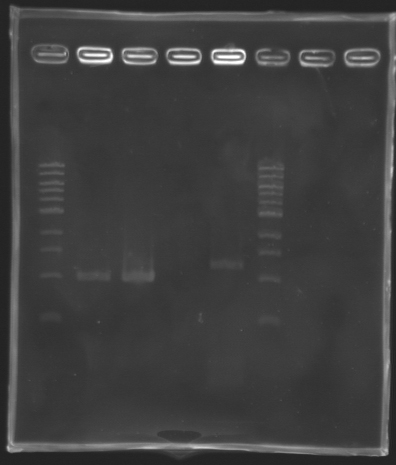
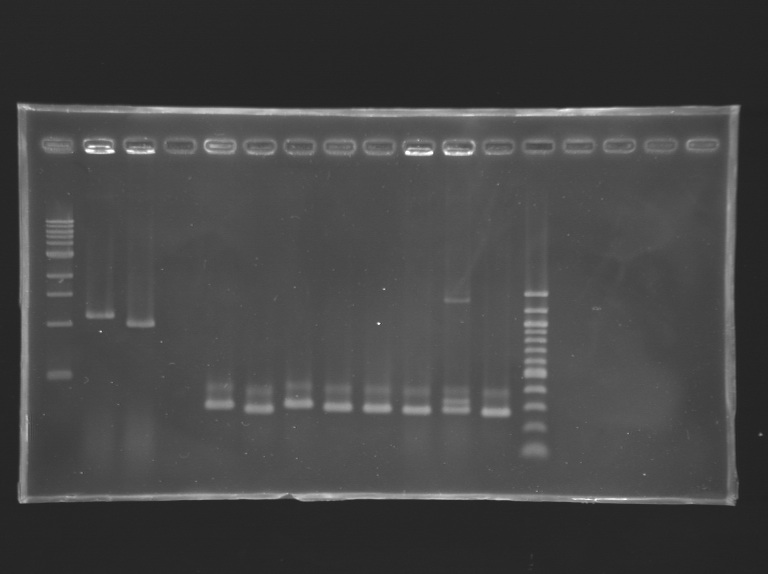
'''Settings Gel 1%''' | '''Settings Gel 1%''' | ||
*Ladder 1 kb 10 µL | *Ladder 1 kb 10 µL | ||
*4µL Template DNA + 2µL Loading Blue | *4µL Template DNA + 2µL Loading Blue | ||
*1 % Agar | *1 % Agar | ||
| + | |||
{| style="text-align: center;" border="1" | {| style="text-align: center;" border="1" | ||
| Line 93: | Line 96: | ||
|style="background: #cbff7B"| bp | |style="background: #cbff7B"| bp | ||
|} | |} | ||
| + | |||
| + | |||
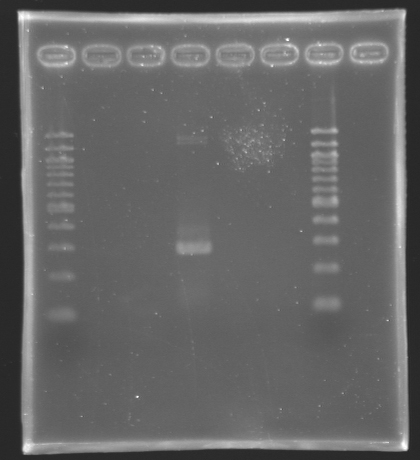
'''Settings Gel 2%''' | '''Settings Gel 2%''' | ||
*Ladder 100 bp 10 µL | *Ladder 100 bp 10 µL | ||
*4µL Template DNA + 2µL Loading Blue | *4µL Template DNA + 2µL Loading Blue | ||
*2 % Agar | *2 % Agar | ||
| + | |||
{| style="text-align: center;" border="1" | {| style="text-align: center;" border="1" | ||
| Line 106: | Line 112: | ||
|'''Measured size''' | |'''Measured size''' | ||
|- | |- | ||
| - | | | + | |PCR_136 |
| - | | | + | |pflhDC (URI) |
|2 | |2 | ||
|197 bp | |197 bp | ||
|style="background: #cbff7B"| ~ 200 bp | |style="background: #cbff7B"| ~ 200 bp | ||
|- | |- | ||
| - | | | + | |PCR_137 |
| - | | | + | |pflhDC (URI) |
| - | | | + | |2 |
| - | | | + | |197 bp |
| - | |style="background: #cbff7B"| | + | |style="background: #cbff7B"| ~ 200 bp |
| + | |- | ||
| + | |PCR_139 | ||
| + | |pflhDC (URI) | ||
| + | |2 | ||
| + | |197 bp | ||
| + | |style="background: #cbff7B"| ~ 200 bp | ||
| + | |- | ||
| + | |PCR_136' | ||
| + | |pflhDC (URI) | ||
| + | |2 | ||
| + | |197 bp | ||
| + | |style="background: #cbff7B"| ~ 200 bp | ||
| + | |- | ||
| + | |PCR_137' | ||
| + | |pflhDC (URI) | ||
| + | |2 | ||
| + | |197 bp | ||
| + | |style="background: #cbff7B"| ~ 200 bp | ||
|} | |} | ||
Revision as of 15:30, 16 August 2008
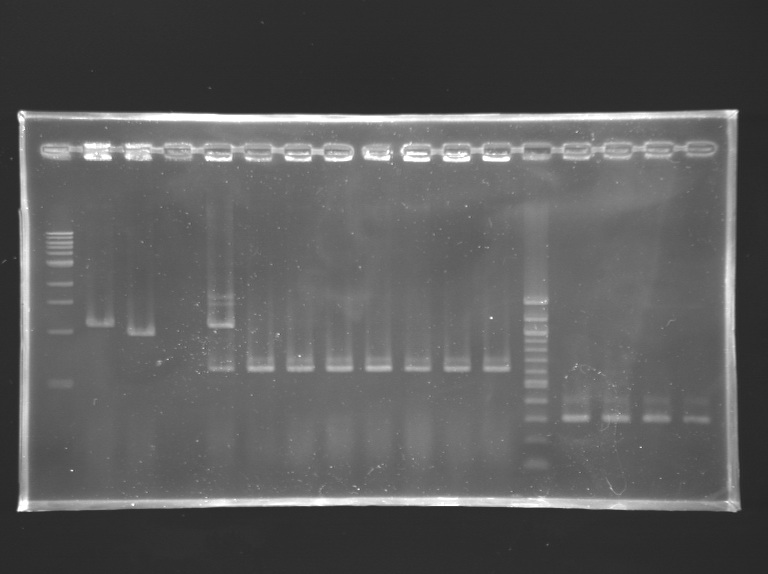
Transformation of the ligations we did yesterdayWe transformed L 143, L 144 and the negative controls T1 and T2, using Invitrogen's TOP10 chemically competent cells standard protocol. PCR amplification of flhDC and its promoterList of PCRs
ProtocolWe followed the standard protocol of amplification in Two steps. PCR program used : PHUSION2 ResultsSettings Gel 1%
DigestionsDigestionMeasurement of concentration of minipreps
DigestionLigation
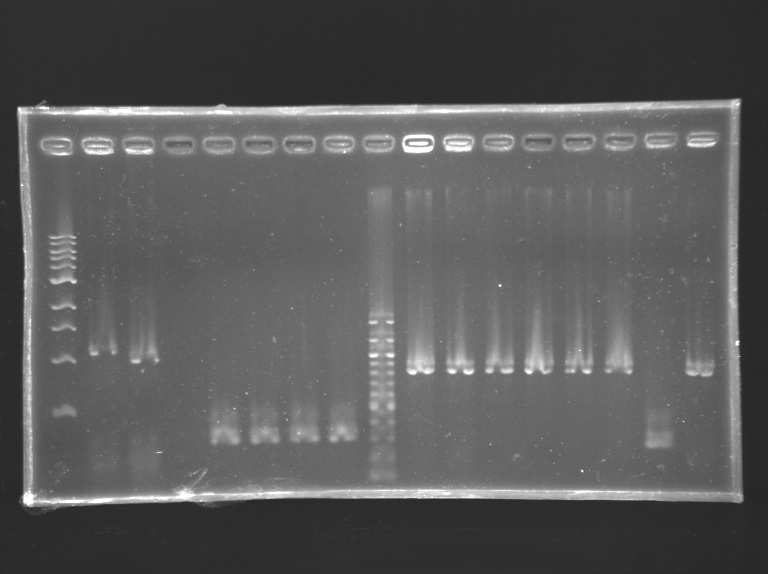
Analysis of yesterday PCR screening
Starting the construction of the Promoter characterization plasmidDigestionMeasurement of concentration of minipreps
Digestion
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"