From 2008.igem.org
(Difference between revisions)
|
|
| Line 169: |
Line 169: |
| | |L164 | | |L164 |
| | |D168 | | |D168 |
| - | | | + | |1.67 |
| | |D132 | | |D132 |
| - | | | + | |2.04 |
| | |- | | |- |
| | |Control L164 | | |Control L164 |
| | |D168 | | |D168 |
| - | | | + | |1.67 |
| | | - | | | - |
| | | - | | | - |
Revision as of 14:59, 23 August 2008
|
← Yesterday ↓ Calendar ↑Tomorrow →
Analysis of the transformant of FlhDC+promotor
- Analysis of the plasmids MP160.1 and MP160.2 (FlhDC+promotor in pSB1A2)
- Control: MP143 (GFP generator in pSB1A2)
PCR
PCR screening programm
elongation time: 1 min 30
total volume reaction (25 µL)
- 12,5 µL Quick load PCR Mix 2X
- 0,5 µL O18
- 0,5 µL O19
- 1 µL DNA
- 10,5 µL water
Digestion
total volume reaction (30 µL)
- 2 µL DNA
- 3 µL buffer 2 10X
- 0,3 µL BSA 100X
- 1 µL XbaI
- 1 µL SpeI
- 22,7 µL water
Incubation 2h55 at 37°C and then 20 min at 65°C.
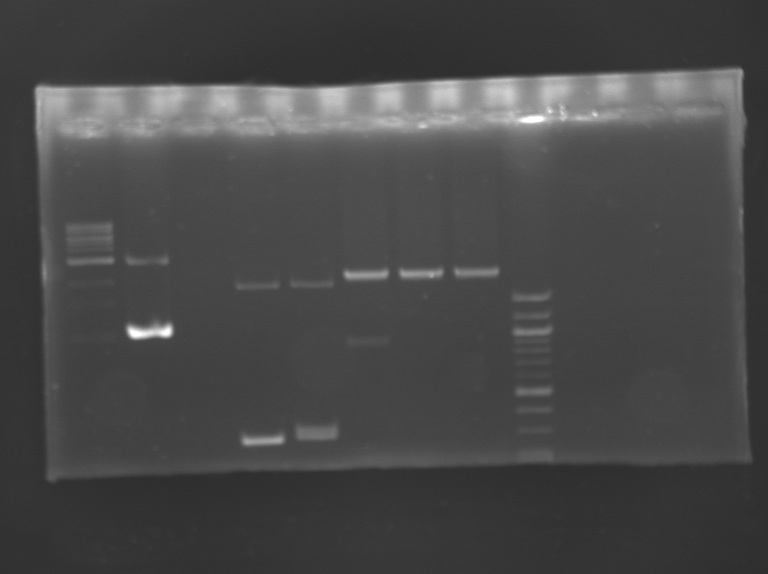
Electrophoresis
- 1% agarose gel
- for PCR products: 10 µL loaded
- for digestion products (30 µL): adding of 7 µL of loading blue and then 20 µL loaded on gel
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
| 9
|
| method
|
| PCR
| digestion
|
|
| sample
| 1 kb DNA ladder
| positive control MP143
| negative control
| MP160.1
| MP160.2
| MP143
| MP160.1
| MP160.2
| 100 bp DNA ladder
|
| expected size
|
| 1114 bp
|
| 1403 bp
| 876 bp (E0240)
2079 (pSB1A2)
| 1165 bp (FlhDC+promotor)
2079 bp (pSB1A2)
|
|
| measured size
|
| 1 kb
3 kb
|
| 0,3 kb
1,8 kb
| 0,3 kb
1,8 kb
| 0,9 kb
2 kb
| 2 kb
| 2 kb
|
|
Results: The clones tested didn't have the insert.
Construction of pFlgA - GFP Generator
Aim : Construction of "pFlgA-RBS-GFP-dbl ter" (pFlgA-E0430/E0432)     
Digestion
Digestion
Protocol Digestion
| Name
| Template DNA
| Description
| Vol MP (µl)
| Vol H2O (µl)
| Enzymes
|
| D168
| MP143.1
| RBS - GFP - term (FV)
| 7.25
| 17.45
| EcoRI and XbaI
|
Protocol
| Well
| 1
| 2
| 3
| 4
| 5
| 6
|
| Sample
| 100 pb ladder
| MP143.1
| no sample
| D168
| no sample
|
| Expected size (pb)
|
|
|
|
|
| Measured size (pb)
|
|
|
|
|
|
Measurement of the concentration of D168 purified
Protocol (it's same that for Miniprep)
| Digestion Name
| Concentration (µg/mL)
| Ratio 260/280
|
| D168
| 24
| 2.15
|
Ligation
Protocol
| Ligation Name
| Vector Name
| Volume Vector (µL)
| Insert
| Volume Insert (µL)
|
| L164
| D168
| 1.67
| D132
| 2.04
|
| Control L164
| D168
| 1.67
| -
| -
|
Construction for FIFO
Aim : Construction of pFlgA - YFP tripart (+/- LVA) "pFlgA-RBS-YFP-dbl ter" (pFlgA-E0430/E0432)     
Transformation of the ligations we did yesterday
Protocol
| Ligation Name
| Description
| Antibio
|
| L160
| D166 (FV) + D132 (FI)
| Amp
|
| Control L160
| D166
| Amp
|
| L161
| D167 (FV) + D1132 (FI)
| Amp
|
| Control L161
| D167
| Amp
|
Construction for synchronization
Transformation of the ligations we did yesterday
Protocol
| Ligation Name
| Description
| Antibio
|
| L158
| D110 (BV) + D131 (BI)
| Amp
|
| Control L158
| D110
| Amp
|
| L159
| D125 (FV) + D109 (FI)
| Kan
|
| Control L159
| D125
| Kan
|
Ligation
Protocol
| Ligation Name
| Description
| Vector Name
| Volume vector (µL)
| Insert Name
| Volume insert (µL)
|
| L162
| rbs-lasI + gfp generator
     
| D107 (BV)
| 10
| D163 (BI)
| 3.46
|
| Control L162
| autoligation control
| D107 (BV)
| 10
| -
| -
|
| L163
| rbs-TetR + gfp generator
     
| D110 (BV)
| 2.5
| D163 (BI)
| 3.44
|
| Control L163
| autoligation control
| D110 (BV)
| 2.5
| -
| -
|
Transformation
Protocol
| Ligation Name
| Description
| Antibio
|
| L162
| D107 (BV) + D163 (BI)
| Amp
|
| Control L162
| D107
| Amp
|
| L163
| D110 (BV) + D163 (BI)
| Amp
|
| Control L163
| D110
| Amp
|
|
 "
"