Team:Paris/August 22
From 2008.igem.org
(Difference between revisions)
AnaJimenez (Talk | contribs) (→Transformation of ligations from August 20th) |
(→Transformation of ligations from August 20th) |
||
| Line 306: | Line 306: | ||
==Transformation of ligations from August 20th== | ==Transformation of ligations from August 20th== | ||
| + | [[Team:Paris/Notebook/Protocols#Transformation |Protocol]] | ||
| - | Top 10 cells were used | + | '''Top 10 cells were used''' |
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
{|border="1" style="text-align: center" | {|border="1" style="text-align: center" | ||
|'''Ligation name''' | |'''Ligation name''' | ||
Revision as of 14:30, 6 September 2008
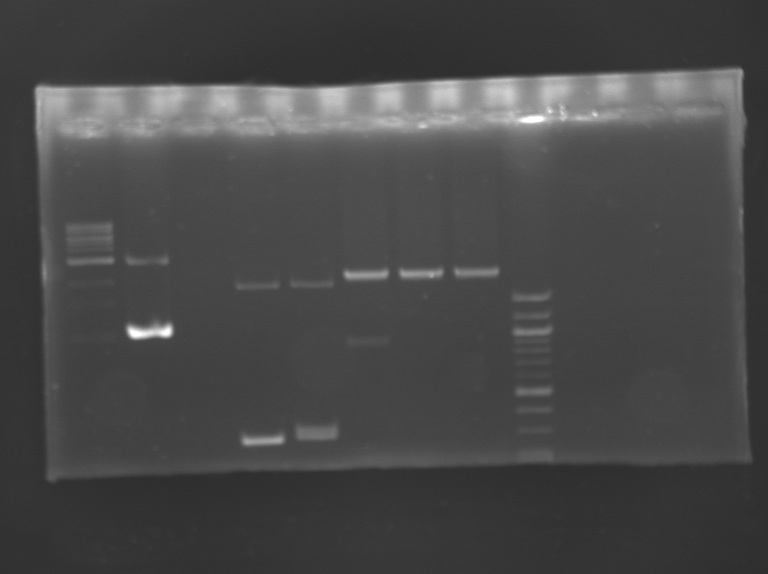
Analysis of the transformant of FlhDC+promotor
PCRPCR screening programm
Digestiontotal volume reaction (30 µL)
Incubation 2h55 at 37°C and then 20 min at 65°C. Electrophoresis
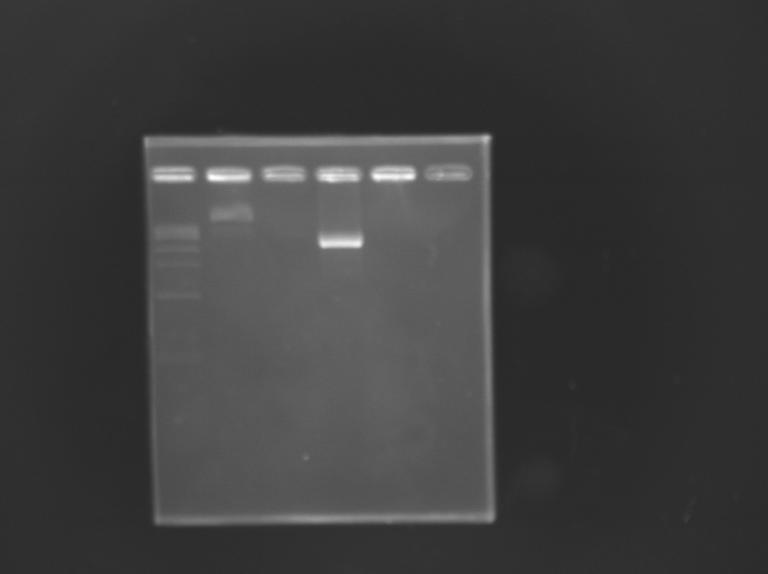
Construction of pFlgA - GFP GeneratorAim : Construction of "pFlgA-RBS-GFP-dbl ter" (pFlgA-E0240) DigestionDigestion
Gel Extraction
Measurement of the concentration of D168 purifiedProtocol (it's same that for Miniprep)
Ligation
Construction for FIFOAim : Construction of pFlgA - YFP tripart (+/- LVA) "pFlgA-RBS-YFP-dbl ter" (pFlgA-E0430/E0432) Transformation of the ligations we did yesterday
Construction for synchronizationTransformation of the ligations we did yesterday
LigationTransformation
Promoter characterization plasmidsTransformation of ligations from August 20thTop 10 cells were used
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"