Team:Paris/August 7
From 2008.igem.org
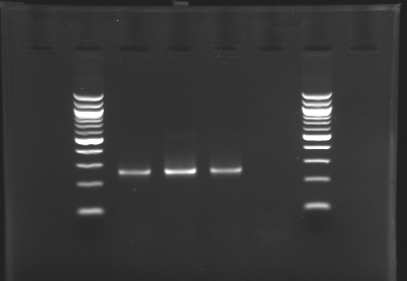
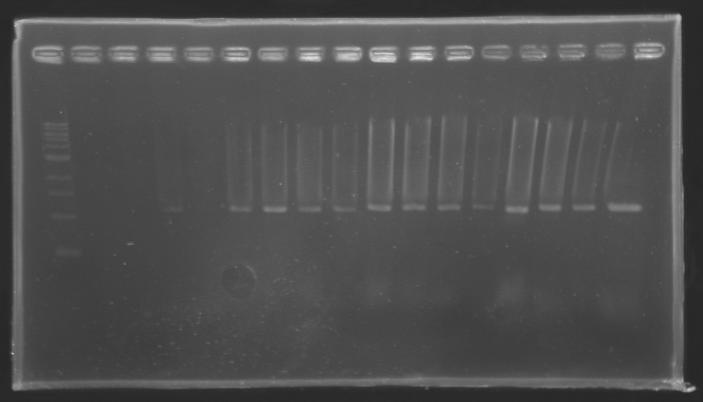
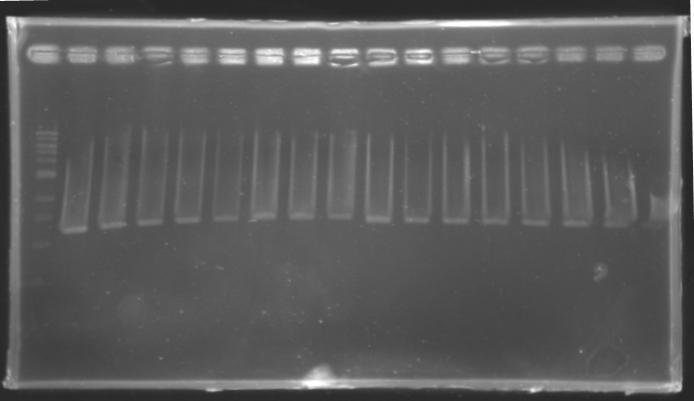
Result of the isolation of coloniesE0240 and pSB3K3 are ok : there is a lot of single colonies S120 and S121 : there is a problem, there is nothing on the plates. We have to check whether those strains are really resistant to Amp. Results of the PCR we did last night
TransformationsProtocolUse of TOP10 chemically competentcells
List of the Ligation Transformation
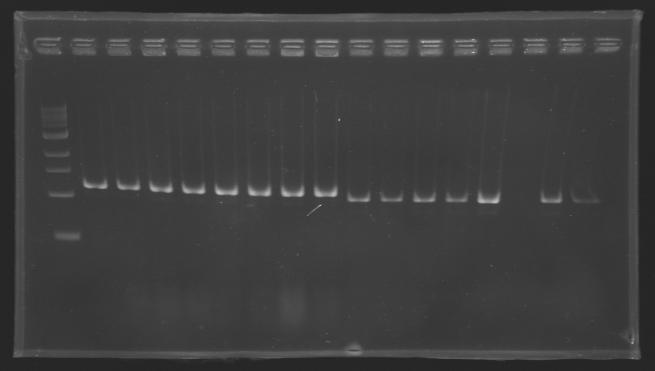
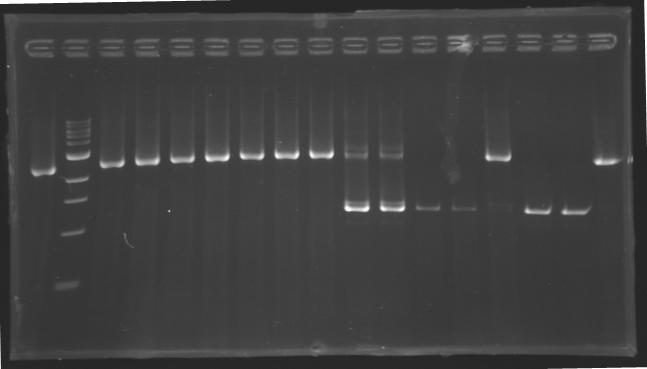
PCR Screening of Ligation Transformants of 1st AugustUse of 8 clones of Ligation transformants for screening PCR
Protocol of screening PCR
Conditions of electrophoresis
Results
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"