Team:Paris/July 17
From 2008.igem.org
(Difference between revisions)
(→Results) |
(→Screening PCR of the transformations) |
||
| (7 intermediate revisions not shown) | |||
| Line 105: | Line 105: | ||
| - | == | + | ==PCR Screening of the transformants== |
Use of the same 8 clones of Biobricks that have been tested for cloning | Use of the same 8 clones of Biobricks that have been tested for cloning | ||
| Line 114: | Line 114: | ||
* 50µl of Mix PCR by tubes | * 50µl of Mix PCR by tubes | ||
* 1 clone | * 1 clone | ||
| - | * | + | * Program : 105°C - for 29 cycles : 95°C; 5' - 95°C; 30" - 55°C; 30" - 72°C; 1,5' |
| - | + | ||
| - | + | ||
===Results=== | ===Results=== | ||
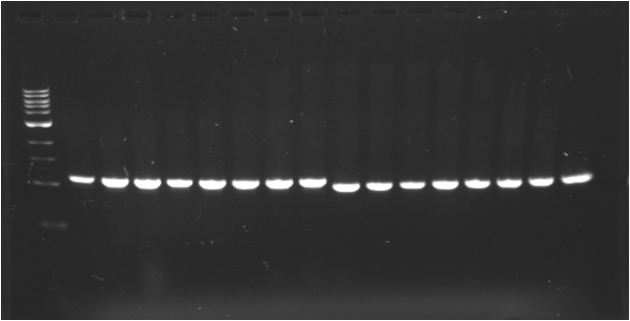
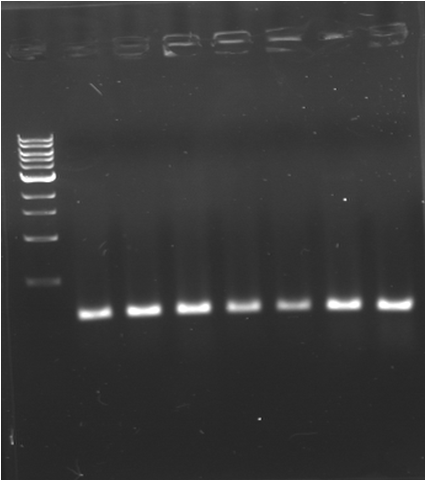
| - | * '''PCR | + | * '''PCR 1 : J23109 (A)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_1| Title = J23109 (A) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 137: | Line 135: | ||
|v_oligoF | |v_oligoF | ||
|10µM 1µL | |10µM 1µL | ||
| - | | | + | |1000pb |
|- | |- | ||
|55°C | |55°C | ||
| Line 181: | Line 179: | ||
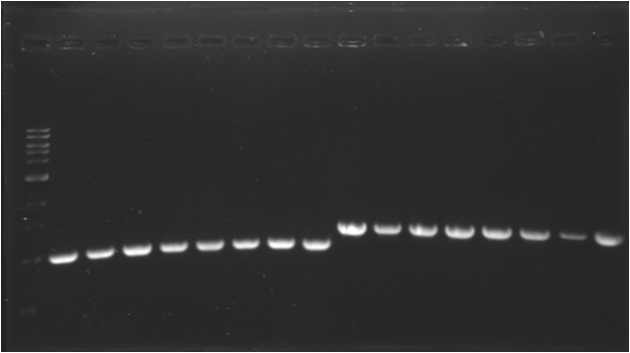
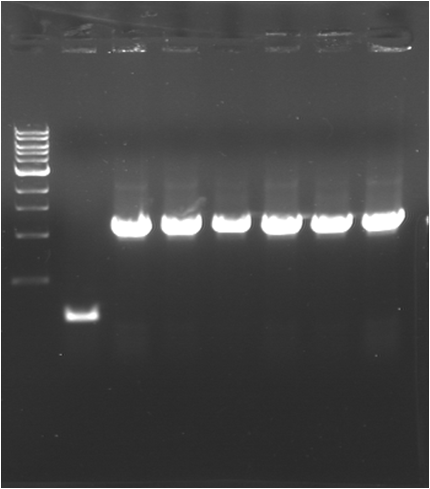
| - | * '''PCR | + | * '''PCR 2 : E0030 (K)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_2| Title = E0030 (K) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 242: | Line 240: | ||
| - | * '''PCR | + | * '''PCR 3 : pSB1A3 (A)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_3| Title = pSB1A3 (A) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 303: | Line 301: | ||
| - | * '''PCR | + | * '''PCR 4 : C0179 (A)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_4| Title = C0179 (A) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 364: | Line 362: | ||
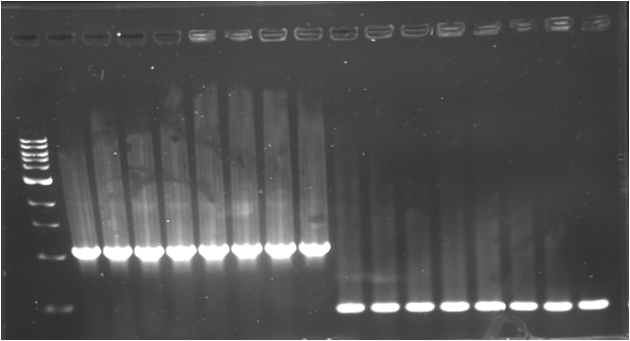
| - | * '''PCR | + | * '''PCR 5 : C0079 (K)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_5| Title = C0079 (K) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 425: | Line 423: | ||
| - | * '''PCR | + | * '''PCR 6 : R0079 (A)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_6| Title = R0079 (A) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 487: | Line 485: | ||
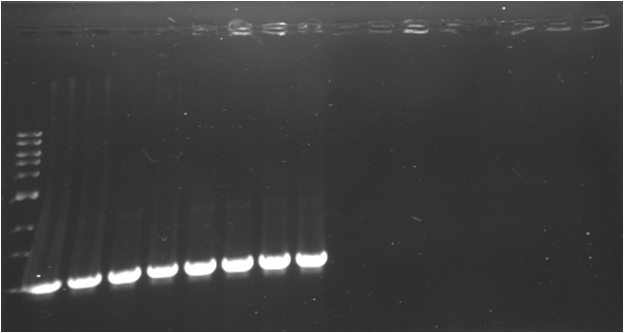
| - | * '''PCR | + | * '''PCR 7 : E1010 (K)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_7| Title = E1010 (K) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 548: | Line 546: | ||
| - | * '''PCR | + | * '''PCR 8 : B0034 (A)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_8| Title = B0034 (A) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 565: | Line 563: | ||
|v_oligoF | |v_oligoF | ||
|10µM 1µL | |10µM 1µL | ||
| - | | | + | | |
|- | |- | ||
|55°C | |55°C | ||
| Line 589: | Line 587: | ||
| | | | ||
| | | | ||
| - | |Image see bands | + | |Image see bands 1 to 7 |
|- | |- | ||
|Number cycles | |Number cycles | ||
| Line 597: | Line 595: | ||
| | | | ||
| | | | ||
| - | |[[Image:080717- | + | |[[Image:080717-5.png|thumb|B0034]] |
|- | |- | ||
|29 | |29 | ||
| Line 609: | Line 607: | ||
| - | * '''PCR : J23101 (A)''' | + | * '''PCR 9 : J23101 (A)''' |
| - | {|border="1" | + | {|border="1" Paris_PCR_9| Title = J23101 (A) |
|'''PCRs setting''' | |'''PCRs setting''' | ||
| | | | ||
| Line 626: | Line 624: | ||
|v_oligoF | |v_oligoF | ||
|10µM 1µL | |10µM 1µL | ||
| - | | | + | |1000pb |
|- | |- | ||
|55°C | |55°C | ||
| Line 650: | Line 648: | ||
| | | | ||
| | | | ||
| - | |Image see bands | + | |Image see bands 2 to 7 |
|- | |- | ||
|Number cycles | |Number cycles | ||
| Line 658: | Line 656: | ||
| | | | ||
| | | | ||
| - | |[[Image:080717- | + | |[[Image:080717-6.png|thumb|J23101]] |
|- | |- | ||
|29 | |29 | ||
| Line 670: | Line 668: | ||
| - | + | * '''PCR 10 : E0040 (A)''' | |
| - | [[Image:080717- | + | {|border="1" Paris_PCR_10| Title = E0040 (A) |
| + | |'''PCRs setting''' | ||
| + | | | ||
| + | |Quick Load | ||
| + | |25µL | ||
| + | | | ||
| + | | | ||
| + | |Expected size | ||
| + | |- | ||
| + | |Annealing | ||
| + | | | ||
| + | |n_oligoF | ||
| + | |18 VF2 | ||
| + | |v_oligoF | ||
| + | |10µM 1µL | ||
| + | |840pb | ||
| + | |- | ||
| + | |55°C | ||
| + | | | ||
| + | |n_oligoR | ||
| + | |19 VR | ||
| + | |v_oligoR | ||
| + | |10µM 1µL | ||
| + | |Sucess | ||
| + | |- | ||
| + | |Time Elongation | ||
| + | | | ||
| + | |Water | ||
| + | |23µL | ||
| + | | | ||
| + | | | ||
| + | |Yes | ||
| + | |- | ||
| + | |1m30' | ||
| + | | | ||
| + | |DNA | ||
| + | |Toothpick of E0040 | ||
| + | | | ||
| + | | | ||
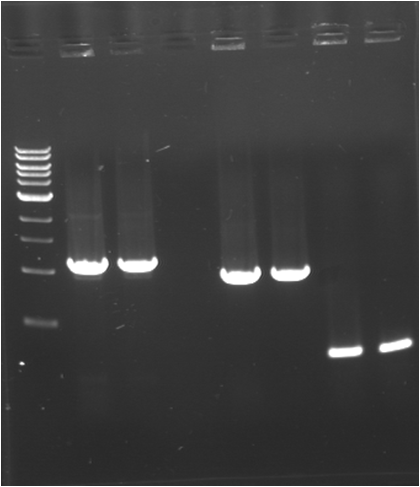
| + | |Image see bands 4 to 5 | ||
| + | |- | ||
| + | |Number cycles | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |[[Image:080717-7.png|thumb|E0040]] | ||
| + | |- | ||
| + | |29 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Ladder band 0 | ||
| + | |} | ||
| - | [[Image:080717-7.png]] | + | |
| + | |||
| + | * '''PCR 11 : R0040 (A)''' | ||
| + | |||
| + | {|border="1" Paris_PCR_11| Title = R0040 (A) | ||
| + | |'''PCRs setting''' | ||
| + | | | ||
| + | |Quick Load | ||
| + | |25µL | ||
| + | | | ||
| + | | | ||
| + | |Expected size | ||
| + | |- | ||
| + | |Annealing | ||
| + | | | ||
| + | |n_oligoF | ||
| + | |18 VF2 | ||
| + | |v_oligoF | ||
| + | |10µM 1µL | ||
| + | |174pb | ||
| + | |- | ||
| + | |55°C | ||
| + | | | ||
| + | |n_oligoR | ||
| + | |19 VR | ||
| + | |v_oligoR | ||
| + | |10µM 1µL | ||
| + | |Sucess | ||
| + | |- | ||
| + | |Time Elongation | ||
| + | | | ||
| + | |Water | ||
| + | |23µL | ||
| + | | | ||
| + | | | ||
| + | |Yes | ||
| + | |- | ||
| + | |1m30' | ||
| + | | | ||
| + | |DNA | ||
| + | |Toothpick of R0040 | ||
| + | | | ||
| + | | | ||
| + | |Image see bands 7 to 8 | ||
| + | |- | ||
| + | |Number cycles | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |[[Image:080717-7.png|thumb|R0040]] | ||
| + | |- | ||
| + | |29 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Ladder band 0 | ||
| + | |} | ||
Latest revision as of 14:35, 31 July 2008
|
Results of the transformations (Biobricks 2007)Legende :
Cloning of the transformations
PCR Screening of the transformantsUse of the same 8 clones of Biobricks that have been tested for cloning
Protocol
Results
|
 "
"