Team:Paris/July 26
From 2008.igem.org
(Difference between revisions)
(→Extraction of the DNA) |
|||
| (9 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== MiniPreps == | == MiniPreps == | ||
| - | * | + | |
| + | * Use of Promega's protocol on all the clones cultivated on the 25th. | ||
| + | * Preparation of 50µl of minipreps in double. | ||
| + | |||
{| border="1" | {| border="1" | ||
| Line 96: | Line 99: | ||
===Digestion Mix=== | ===Digestion Mix=== | ||
| - | 10µl of Miniprep ( | + | 10µl of Miniprep (26 aug.) <br> |
12.5µl of water<br> | 12.5µl of water<br> | ||
2.5µl of Buffer N°2<br> | 2.5µl of Buffer N°2<br> | ||
| Line 102: | Line 105: | ||
1µl of enzyme 1<br> | 1µl of enzyme 1<br> | ||
1µl of enzyme 2<br> | 1µl of enzyme 2<br> | ||
| + | |||
| + | * Incubation 1h at 37°C with the first enzyme | ||
| + | * Add the second enzyme | ||
| + | * Incubation 1h at 37°C with the second enzyme | ||
| + | * Store on ice | ||
| + | * Revelation of the digestion by electrophoresis on agarose gel | ||
| + | |||
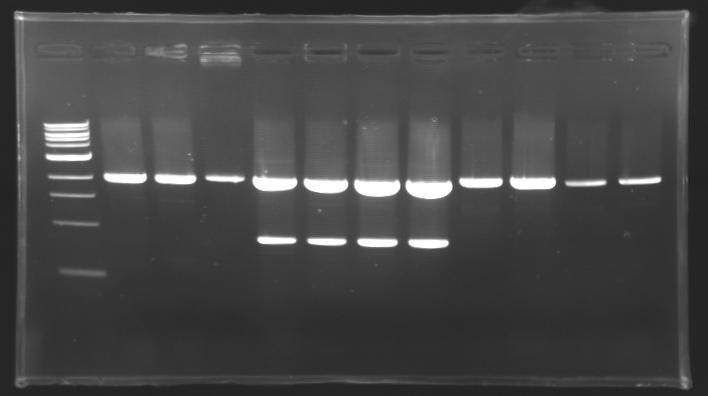
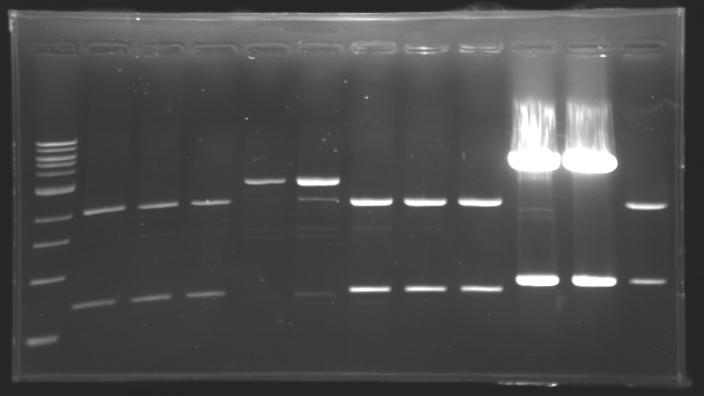
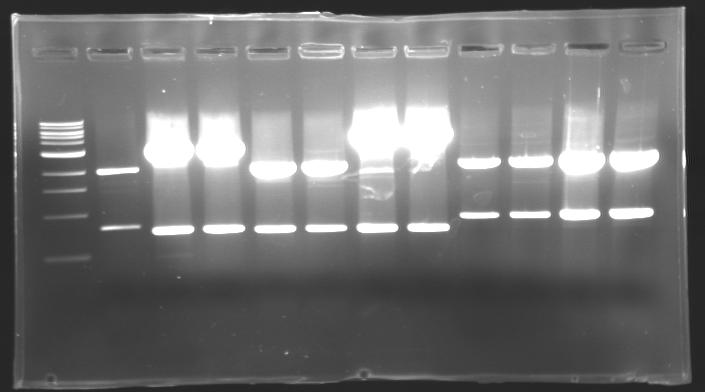
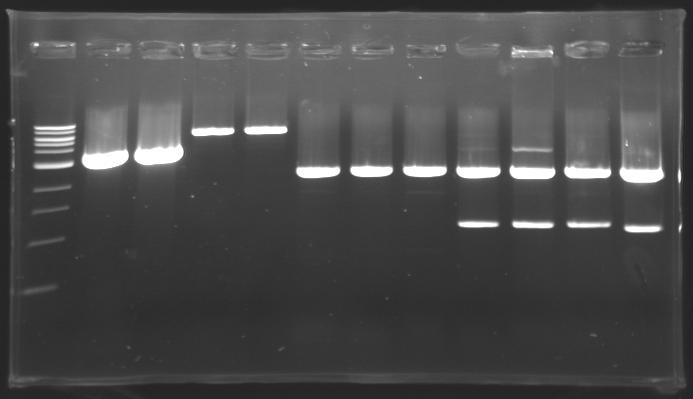
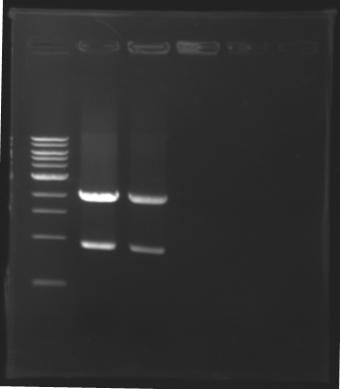
=== Results of digestions : Electrophoresis === | === Results of digestions : Electrophoresis === | ||
| - | Gel 1, 2, 3, 4, 5 = 0.8% | + | '''conditions :''' |
| + | |||
| + | * 10µl of ladder 1 kb (except for gel n°6 : 100 pb) | ||
| + | * 30µl of digestion added with 5µl of loading Dye 6x | ||
| + | * migration ~30min at 100W | ||
| + | * Gel 1, 2, 3, 4, 5 = '''0.8%''' | ||
| + | * Gel 6 = '''1,5%''' | ||
| Line 115: | Line 131: | ||
| - | gel5 [[Image: | + | gel5 [[Image:080726gel_5.jpg| gel5|100px]] |
| - | gel6 [[Image: | + | gel6 [[Image:080726gel_6.jpg| gel6|100px]] |
| - | {| border="1" | + | {|- border="1" |
| - | |align=center|'''Name''' | + | |align=center style="background: #649CD7;"|'''Name''' |
| - | |align=center|'''BioBrick''' | + | |align=center style="background: #649CD7;"|'''BioBrick''' |
| - | |align=center|'''Tube N°''' | + | |align=center style="background: #649CD7;"|'''Tube N°''' |
| - | |align=center|'''Enz 1''' | + | |align=center style="background: #649CD7;"|'''Enz 1''' |
| - | |align=center|'''Enz 2''' | + | |align=center style="background: #649CD7;"|'''Enz 2''' |
| - | |align=center|'''Obs''' | + | |align=center style="background: #649CD7;"|'''Obs''' |
| - | |align=center|'''Exp Size Matrix''' | + | |align=center style="background: #649CD7;"|'''Exp Size Matrix''' |
| - | |align=center|'''Exp Size BB''' | + | |align=center style="background: #649CD7;"|'''Exp Size BB''' |
| - | |align=center|'''Mea Size Matrix''' | + | |align=center style="background: #649CD7;"|'''Mea Size Matrix''' |
| - | |align=center|'''Mea Size BB''' | + | |align=center style="background: #649CD7;"|'''Mea Size BB''' |
| - | |align=center|'''Gel''' | + | |align=center style="background: #649CD7;"|'''Gel''' |
| - | |align=center|'''Band''' | + | |align=center style="background: #649CD7;"|'''Band''' |
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="2" style="background: #D4E2EF;" | D100 |
| - | ! rowspan=" | + | ! rowspan="2" style="background: #D4E2EF;" | B0034 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| XbaI | ! rowspan="2"| XbaI | ||
| Line 151: | Line 167: | ||
! rowspan="1"| 3 | ! rowspan="1"| 3 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D101 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | B0034 | ||
|align=center|3 | |align=center|3 | ||
|align=center| EcoRI | |align=center| EcoRI | ||
| Line 162: | Line 180: | ||
|align=center| 2 | |align=center| 2 | ||
|- | |- | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | D102 | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | B0034 | ||
|align=center|4 | |align=center|4 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 177: | Line 197: | ||
|align=center| 4 | |align=center| 4 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | D103 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | J23101 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 194: | Line 214: | ||
|align=center| 6 | |align=center| 6 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | D104 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | J23109 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 211: | Line 231: | ||
! rowspan="1"| 8 | ! rowspan="1"| 8 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | D105 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | R0079 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 228: | Line 248: | ||
|align=center| 10 | |align=center| 10 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | D106 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;" | R0040 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 245: | Line 265: | ||
|align=center| 12 | |align=center| 12 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;" | D107 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;" | S03154 |
|align=center|1 | |align=center|1 | ||
! rowspan="1"| SpeI | ! rowspan="1"| SpeI | ||
| Line 258: | Line 278: | ||
|align=center| 5 | |align=center| 5 | ||
|- | |- | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | D108 | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | S03154 | ||
|align=center|2 | |align=center|2 | ||
! rowspan="2"| XbaI | ! rowspan="2"| XbaI | ||
| Line 273: | Line 295: | ||
|align=center| 3 | |align=center| 3 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D109 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | S03154 | ||
|align=center|4 | |align=center|4 | ||
| align=center|EcoRI | | align=center|EcoRI | ||
| Line 284: | Line 308: | ||
|align=center| 4 | |align=center| 4 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;" | D110 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;" | S03879 |
|align=center|1 | |align=center|1 | ||
| align=center|SpeI | | align=center|SpeI | ||
| Line 297: | Line 321: | ||
|align=center| 6 | |align=center| 6 | ||
|- | |- | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | D111 | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | S03879 | ||
|align=center|2 | |align=center|2 | ||
! rowspan="2"| XbaI | ! rowspan="2"| XbaI | ||
| Line 312: | Line 338: | ||
|align=center| 8 | |align=center| 8 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D112 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | S03879 | ||
|align=center|4 | |align=center|4 | ||
| align=center|EcoRI | | align=center|EcoRI | ||
| Line 323: | Line 351: | ||
|align=center| 9 | |align=center| 9 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;" |D113 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;" |C0079 |
|align=center| 1 | |align=center| 1 | ||
|align=center|EcoRI | |align=center|EcoRI | ||
| Line 336: | Line 364: | ||
|align=center| 10 | |align=center| 10 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D114 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | C0079 | ||
|align=center| 2 | |align=center| 2 | ||
|align=center|XbaI | |align=center|XbaI | ||
| Line 347: | Line 377: | ||
|align=center| 11 | |align=center| 11 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|D115 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|C0179 |
|align=center| 1 | |align=center| 1 | ||
|align=center| EcoRI | |align=center| EcoRI | ||
| Line 360: | Line 390: | ||
|align=center| 12 | |align=center| 12 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D116 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | C0179 | ||
|align=center| 2 | |align=center| 2 | ||
|align=center| XbaI | |align=center| XbaI | ||
| Line 371: | Line 403: | ||
|align=center| 2 | |align=center| 2 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|D117 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|E0030 |
|align=center| 1 | |align=center| 1 | ||
|align=center|EcoRI | |align=center|EcoRI | ||
| Line 384: | Line 416: | ||
|align=center| 3 | |align=center| 3 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D118 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | E0030 | ||
|align=center| 2 | |align=center| 2 | ||
|align=center| XbaI | |align=center| XbaI | ||
| Line 395: | Line 429: | ||
|align=center| 4 | |align=center| 4 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|D119 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|E0040 |
|align=center| 1 | |align=center| 1 | ||
|align=center| EcoRI | |align=center| EcoRI | ||
| Line 408: | Line 442: | ||
|align=center| 5 | |align=center| 5 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D120 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | E0040 | ||
|align=center| 2 | |align=center| 2 | ||
|align=center| XbaI | |align=center| XbaI | ||
| Line 419: | Line 455: | ||
|align=center| 6 | |align=center| 6 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|D121 |
| - | ! rowspan=" | + | ! rowspan="1" style="background: #D4E2EF;"|E1010 |
|align=center| 1 | |align=center| 1 | ||
|align=center| EcoRI | |align=center| EcoRI | ||
| Line 432: | Line 468: | ||
|align=center| 7 | |align=center| 7 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D122 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | E1010 | ||
|align=center| 2 | |align=center| 2 | ||
|align=center| XbaI | |align=center| XbaI | ||
| Line 443: | Line 481: | ||
|align=center| 8 | |align=center| 8 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|D123 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|J23100 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 460: | Line 498: | ||
! rowspan="1"| 10 | ! rowspan="1"| 10 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|D124 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|J23107 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 477: | Line 515: | ||
! rowspan="1"| 12 | ! rowspan="1"| 12 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|D125 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|B0015 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"|EcoRI | ! rowspan="2"|EcoRI | ||
! rowspan="2"|XbaI | ! rowspan="2"|XbaI | ||
| - | ! rowspan="2"| | + | ! rowspan="2"| BV |
! rowspan="2"| 3303 pb | ! rowspan="2"| 3303 pb | ||
! rowspan="2"| 15 pb | ! rowspan="2"| 15 pb | ||
| Line 494: | Line 532: | ||
! rowspan="1"| 3 | ! rowspan="1"| 3 | ||
|- | |- | ||
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|D126 |
| - | ! rowspan="2" style="background: # | + | ! rowspan="2" style="background: #D4E2EF;"|I0500 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"|SpeI | ! rowspan="2"|SpeI | ||
| Line 511: | Line 549: | ||
! rowspan="1"| 5 | ! rowspan="1"| 5 | ||
|- | |- | ||
| - | ! rowspan=" | + | ! rowspan="2" style="background: #D4E2EF;" | D127 |
| - | ! rowspan=" | + | ! rowspan="2" style="background: #D4E2EF;" | B0030 |
|align=center|1 | |align=center|1 | ||
! rowspan="2"| XbaI | ! rowspan="2"| XbaI | ||
| Line 528: | Line 566: | ||
! rowspan="1"| 5 | ! rowspan="1"| 5 | ||
|- | |- | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | D128 | ||
| + | ! rowspan="1" style="background: #D4E2EF;" | B0030 | ||
|align=center|3 | |align=center|3 | ||
! rowspan="1"| EcoRI | ! rowspan="1"| EcoRI | ||
| Line 539: | Line 579: | ||
! rowspan="1"| 6 | ! rowspan="1"| 6 | ||
|- | |- | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | D129 | ||
| + | ! rowspan="2" style="background: #D4E2EF;" | B0030 | ||
|align=center|4 | |align=center|4 | ||
! rowspan="2"| SpeI | ! rowspan="2"| SpeI | ||
| Line 554: | Line 596: | ||
! rowspan="1"| 8 | ! rowspan="1"| 8 | ||
|- | |- | ||
| - | ! rowspan="3" style="background: # | + | ! rowspan="3" style="background: #D4E2EF;"|D130 |
| - | ! rowspan="3" style="background: # | + | ! rowspan="3" style="background: #D4E2EF;"|E0422 |
|align=center|1 | |align=center|1 | ||
! rowspan="3"| XbaI | ! rowspan="3"| XbaI | ||
| Line 575: | Line 617: | ||
! rowspan="1"| 11 | ! rowspan="1"| 11 | ||
|- | |- | ||
| - | ! rowspan="3" style="background: # | + | ! rowspan="3" style="background: #D4E2EF;"|D131 |
| - | ! rowspan="3" style="background: # | + | ! rowspan="3" style="background: #D4E2EF;"|E0840 |
|align=center|1 | |align=center|1 | ||
! rowspan="3"| XbaI | ! rowspan="3"| XbaI | ||
| Line 598: | Line 640: | ||
! rowspan="1"| 3 | ! rowspan="1"| 3 | ||
|} | |} | ||
| + | |||
| + | |||
| + | ==> conclusion : all the digestion have succeed.....GREAT ! | ||
| + | |||
| + | == DNA extraction == | ||
| + | |||
| + | |||
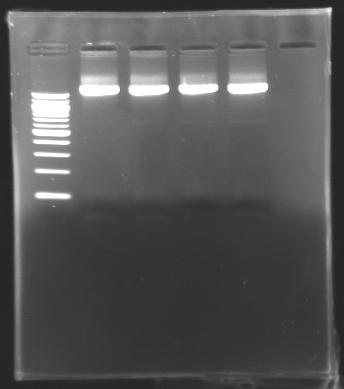
| + | * Cutting of the parts of interest, for all the digestion that have migrated on the gels | ||
| + | * Use of Promega's protocol for the extraction. | ||
| + | * Test of the succeed of the extraction by electrophoresis on 2µl of the parts extracted. | ||
| + | |||
| + | |||
| + | ==> conclusion : we succeed to detect DNA in our samples this time. | ||
Latest revision as of 16:55, 31 July 2008
|
MiniPreps
DigestionDigestion Mix10µl of Miniprep (26 aug.)
Results of digestions : Electrophoresisconditions :
DNA extraction
|
 "
"