Team:Paris/July 29
From 2008.igem.org
(→DNA digestion and purification) |
|||
| Line 200: | Line 200: | ||
|align=center| | |align=center| | ||
! rowspan="1"| 8 & 9 | ! rowspan="1"| 8 & 9 | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == '''Screening PCR of the transformations with Ligation '''== | ||
| + | |||
| + | Use of the same 8 clones of Biobricks that have been tested for cloning | ||
| + | |||
| + | |||
| + | ===Protocol=== | ||
| + | |||
| + | * 50µl of Mix PCR by tubes | ||
| + | * one 1 clone | ||
| + | * Program : 105°C - for 29 cycles : 95°C; 5' - 95°C; 30" - 55°C; 30" - 72°C; 1,5' | ||
| + | |||
| + | ===Results=== | ||
| + | |||
| + | * '''PCR 1 : J23109 (A)''' | ||
| + | |||
| + | {|border="1" Paris_PCR_1| '''PCRs setting''' (A) | ||
| + | | | ||
| + | |Quick Load | ||
| + | |25µL | ||
| + | | | ||
| + | | | ||
| + | |Expected size | ||
| + | |- | ||
| + | |Annealing | ||
| + | | | ||
| + | |n_oligoF | ||
| + | |18 VF2 | ||
| + | |v_oligoF | ||
| + | |10µM 1µL | ||
| + | |1000pb | ||
| + | |- | ||
| + | |55°C | ||
| + | | | ||
| + | |n_oligoR | ||
| + | |19 VR | ||
| + | |v_oligoR | ||
| + | |10µM 1µL | ||
| + | |Sucess | ||
| + | |- | ||
| + | |Time Elongation | ||
| + | | | ||
| + | |Water | ||
| + | |23µL | ||
| + | | | ||
| + | | | ||
| + | |Yes | ||
| + | |- | ||
| + | |1m30' | ||
| + | | | ||
| + | |DNA | ||
| + | |Toothpick of J23109 | ||
| + | | | ||
| + | | | ||
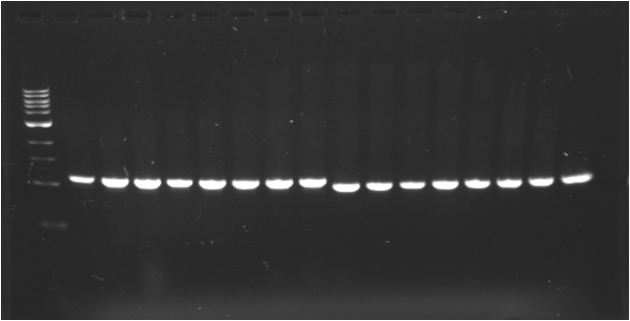
| + | |Image see bands 1 to 8 | ||
| + | |- | ||
| + | |Number cycles | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |[[Image:080717-1.png|thumb|J23109]] | ||
| + | |- | ||
| + | |29 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Ladder band 0 | ||
| + | |} | ||
| + | |||
| + | |||
| + | * '''PCR 2 : E0030 (K)''' | ||
| + | |||
| + | {|border="1" Paris_PCR_2| Title = E0030 (K) | ||
| + | |'''PCRs setting''' | ||
| + | | | ||
| + | |Quick Load | ||
| + | |25µL | ||
| + | | | ||
| + | | | ||
| + | |Expected size | ||
| + | |- | ||
| + | |Annealing | ||
| + | | | ||
| + | |n_oligoF | ||
| + | |18 VF2 | ||
| + | |v_oligoF | ||
| + | |10µM 1µL | ||
| + | |723pb | ||
| + | |- | ||
| + | |55°C | ||
| + | | | ||
| + | |n_oligoR | ||
| + | |19 VR | ||
| + | |v_oligoR | ||
| + | |10µM 1µL | ||
| + | |Sucess | ||
| + | |- | ||
| + | |Time Elongation | ||
| + | | | ||
| + | |Water | ||
| + | |23µL | ||
| + | | | ||
| + | | | ||
| + | |Yes | ||
| + | |- | ||
| + | |1m30' | ||
| + | | | ||
| + | |DNA | ||
| + | |Toothpick of E0030 | ||
| + | | | ||
| + | | | ||
| + | |Image see bands 9 to 16 | ||
| + | |- | ||
| + | |Number cycles | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |[[Image:080717-1.png|thumb|E0030]] | ||
| + | |- | ||
| + | |29 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Ladder band 0 | ||
| + | |} | ||
| + | |||
| + | |||
| + | * '''PCR 3 : pSB1A3 (A)''' | ||
| + | |||
| + | {|border="1" Paris_PCR_3| Title = pSB1A3 (A) | ||
| + | |'''PCRs setting''' | ||
| + | | | ||
| + | |Quick Load | ||
| + | |25µL | ||
| + | | | ||
| + | | | ||
| + | |Expected size | ||
| + | |- | ||
| + | |Annealing | ||
| + | | | ||
| + | |n_oligoF | ||
| + | |18 VF2 | ||
| + | |v_oligoF | ||
| + | |10µM 1µL | ||
| + | | | ||
| + | |- | ||
| + | |55°C | ||
| + | | | ||
| + | |n_oligoR | ||
| + | |19 VR | ||
| + | |v_oligoR | ||
| + | |10µM 1µL | ||
| + | |Sucess | ||
| + | |- | ||
| + | |Time Elongation | ||
| + | | | ||
| + | |Water | ||
| + | |23µL | ||
| + | | | ||
| + | | | ||
| + | |Yes | ||
| + | |- | ||
| + | |1m30' | ||
| + | | | ||
| + | |DNA | ||
| + | |Toothpick of pSB1A3 | ||
| + | | | ||
| + | | | ||
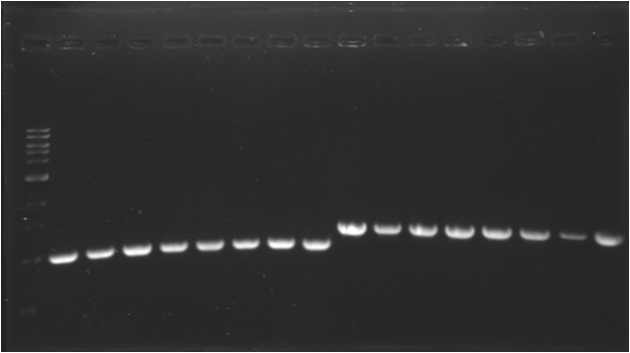
| + | |Image see bands 1 to 8 | ||
| + | |- | ||
| + | |Number cycles | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |[[Image:080717-2.png|thumb|pSB1A3]] | ||
| + | |- | ||
| + | |29 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |Ladder band 0 | ||
|} | |} | ||
Revision as of 09:24, 31 July 2008
DNA digestion and purificationfor each reaction (total volume : 50 µL)
Each reaction was incubated 2 hours at 37°C, then 10 minutes at 60-65°C (to inactivate the enzymes). 10 µL of loading dye (6X) were added to each of the 50 µL of digestion product. The whole samples were run in a 1,5% agarose gel (about 30 minutes at 100 W ; 2 x 30 µL per sample ; 30 µL per well). The bands of interest were then excised from the gel and the DNA was purified using the QIAquick DNA Gel Extraction kit (QIAGEN). Unfortunately, there were not enough columms, so we took some columms from the QIAGEN MiniPrep kit, hoping that it will work with the QIAquick DNA Gel Extraction kit. Some of the samples were too voluminous, so we separated them into two tubes. The elution of DNA was performed using 50 µL of water (after 10 minutes of incubation at 37°C). Each of the samples was then analysed by a 1,5% agarose gel:
The ladder used was the 100 bp ladder from New England Biolabs.
Screening PCR of the transformations with LigationUse of the same 8 clones of Biobricks that have been tested for cloning
Protocol
Results
|
 "
"