|
Home
The Team
Project Report
Parts
Modeling
Notebook
Safety
CoLABoration
|
_august
Aug. 8th 2008
The T-cells B12.7.5 from Mahima
10 ml of the cells were taken from the culture (30 ml) and centrifuged at 1200 rpm for 5 minutes. Then the old medium was replaced by 10 ml of fresh medium, the cells were resupended and transferred to a new dish with 20 ml fresh medium.
The cells have to be split approximately every 3 days (the medium should start to become yellow).
We use RPMI medium containing 10% FCS, HEPES 10mM, ß-Mercaptoethanol 50µM, L-Glutamine 2mM and Pen/Strep 1%.
Aug. 11th 2008
Solving the parts of the IGEM 2008 parts collection and transformation
I warmed 5 µl TE to 50° in PCR tubes. Then I punched out the desired parts (CMV promotor BBa_I712004, transfectionvector BBa_J52017) and put it into the warmed TE puffer, spin the tubes and after 20 minutes I started the transformation with the following protocol
- 2µl of the eluted DNA to the thawed competent cells (RV308)
- 20 minutes on ice
- heat shock 42° for 1 minute
- 5 minutes on ice
- 900µl DyT and incubation at 37° for 70 minutes
- plating out of 50µl and 100µl on LB/Amp plates (1µl Amp stock solution for 1 ml LB)
Aug. 12th 2008
Solving the parts of the IGEM 2008 parts collection and transformation
There were no colonies on the plates so I changed the protocol and tried it again.
I solved the DNA in 10µl warmed TE and incubated at 50° while elution. And the transformation:
- 5µl of the eluted DNA to XL-1 cells
- 30 minutes on ice
- heat shock 42° 1 minute
- 5 minutes on ice
- 900µl DyT and transfering the cells in bigger tubes for a better ventilation for 2 hours at 37°
- plating out of 50µl on LB/Amp plates
- centrifuging for 3 minutes at 1000 rpm
- put away the supernatant and plating out the remaining 100µl
Freezing aliquots of the B.12.7.5 cells from Mahima
- mixing FCS with 15% DMSO
- centrifuging 30ml of the cell suspension 5 minutes at 1200rpm
- taking away the old medium and resuspending the cells in 2.5 ml FCS/DMSO
- making 0.5ml aliquots in special freezing tubes
- freezing at -80°
Aug. 13th 2008
There again were no colonies on the plates.
Preparing T-cells for MTT-assay (Normann)
To test the Mg2+ tolerance of the T-cells 800µl medium was given to 100µl cellsuspension and 100µl MgCl2/MgAc Solution with different concentrations.
well --- Mg2+ [mM]
1 ------- control
2 ------- 12.5
3 ------- 8.75
4 ------- 6.25
5 ------- 5
6 ------- 3.75
7 ------- 2.5
8 ------- 1.25
Aug. 14th 2008
The freezed aliquots of the B.12.7.5 cells
I put the cells in nitrogen. They are located in the tank 1, stock 5, box 6 (the first row with the red caps)
Using the part collection of 2007
- 15µl ddH20 to the wells of the sc-fluorescein(BBa_J07014) and the transfectionvector(BBa_J52017)and puting in an 1.5ml Eppi
- 1µl to XL-1 cells
- 20 minutes on ice
- 1 minute heat shock 42°
- 5 minutes on ice
- 900µl DyT and transfering to bigger tubes
- 70 minutes at 37°
- plating out 50µl on LB/Amp plates and centrifuging the reamining cells at 1000rpm for 3 minutes
- discarding the supernatant and plating out the remaining 100µl
Aug. 15th 2008
There were colonies on the plates from both parts.
Splitting T-cells(Normann)
The cells (15ml) were spun down at 1200 rpm (5min) and resolved with 10ml PBS. Then they were spun down again and resolved in 15ml fresh medium. 5ml was given to a dish and 15ml additional medium was added.
MTT-assay Normann
- centrifuging 1ml cellsuspension at 1200 rpm (5min)-> discarding the supernatant
- adding 200µl fresh medium and 50µl MTT
- waiting for 4h
- centrifuging again at 1200rpm (5min) -> discarding the supernantant
- resolving the pellet with 400µl DMSO and adding 50µl Soerensen
- incubation for 10 min
- measuring at 570nm (autozero with DMSO+Soerensen)
| Mg2+ [mM] |
A (MgCl2) |
A (MgAc) |
| 12,5 |
0,388 |
0,284 |
| 8,75 |
0,488 |
0,23 |
|
| 6,25 |
0,568 |
0,217 |
| 5 |
0,328 |
0,22 |
| 3,75 |
0,365 |
0,311 |
| 2,5 |
0,255 |
0,295 |
| 1,25 |
0,207 |
0,241 |
| control |
0,449 |
0,395 |
Aug. 17th 2008
Picking colonies
I picked three colonies from each plate (sc-fluorescein and transfectionvector), put them into 5ml LB/Amp and incubated at 37° ~16h
Transformation of the parts CMV promotor(BBa_J52034) and CMV+Rluc(BBa_J52038) from 2007
I did the transformation using the protocol from "Using the part collection of 2007"(08-14-2008)
Aug. 18th 2008
Miniprep of the picked colonies (sc-fluorescein and transfectionvector)
I did the Miniprep with the QIAprep Spin MIniprep Kit
Analytic digestion
For the sc-fluorescein I did a double digestion with SpeI and EcoRI:
- 5µl plasmid, 0,5µl SpeI, 0,5µl EcoRI, 0,5µl BSA, 1µl Buffer P2, 2,5µl H2O
- 2,5h at 37°
- 1% agarose gel
For the transfectionvector I did a digestion with EcoRI:
- 5µl plasmid, 1µl EcoRI, 1µl Buffer P2, 3µl H2O
- 2,5h at 37°
- 1% agarose gel
Picking colonies
I picked three colonies from each plate (CMV promotor and CMV+Rluc), put them into 5ml LB/Amp and incubated for ~16h at 37°
Aug. 19th 2008
Analytic digestion
For the CMV promotor and the CMV+Rluc construct I did a double digestion with SpeI and EcoRI:
- 5µl plasmid, 0,5µl SpeI, 0,5µl EcoRI, 0,5µl BSA, 1µl Buffer P2, 2,5µl H2O
- 2,5h at 37°
- 1% agarose gel
Aug. 22nd 2008
Thawing 293T cells
For expressing our receptor constructs we decided to use 293T cells. We wanted to test the efficiency of transfection of the 293T cells, so I thawed the cells and put them in culture. I used DMEM medium with 1% Glutamine, 10%FCS and 1% Pen/Strep. I thawed the cells at 37°C and put them in a cell culture flask with 20 ml medium
Aug. 25th 2008
Splitting the cells
I split the cells and transfered them to a 6 well dish. You should transfer 6*10^4 cells/cm². I counted the cells in the neubauer chamber and counted 15*10000 cells/ml so I transfered 500 µl of the cell suspension in one well.
Aug. 26th 2008
Transfection
One hour before transfection I washed the cells once with PBS and filled up with fresh medium. Normann did the transfection with a plasmid carrying the ß-Galactosidase gene.
Aug. 28th 2008
Harvesting cells
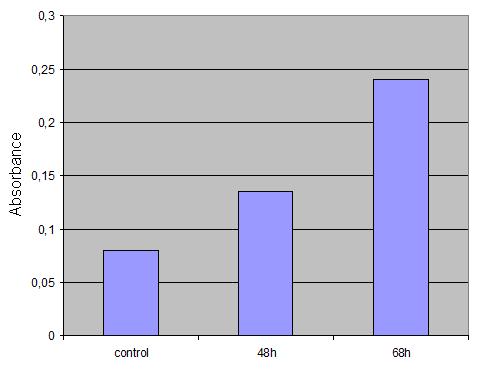
For the ONPG Test we decided to use cells after several timepoints. So I harvested cells after 48h and 68h.
I washed the cells twice with PBS. Then I took 500µl PBS, lost the cells with a cell scraper and transfered it to an eppi. After centrifugation (2min 13000rpm) I replaced the PBS with 500µl 1xLysepuffer, resuspended the pellet by pipetting up and down and put it for 15 min at -80°. Then I thawed the cells, vortexed it, centrifuged again and transfered the supernatant to a fresh eppi. Then I put the supernatant at -20°.
Control was done with untransfected cells using the same procedure.
Result
|