Team:Hawaii/Notebook/2008-07-25
From 2008.igem.org
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Checked Re-streaked Transformation
- Margaret
- The results are here
Plasmid prep
- Grace
- Plasmid prep for nir+B0034, GFP+B0024, GFPf+B0024
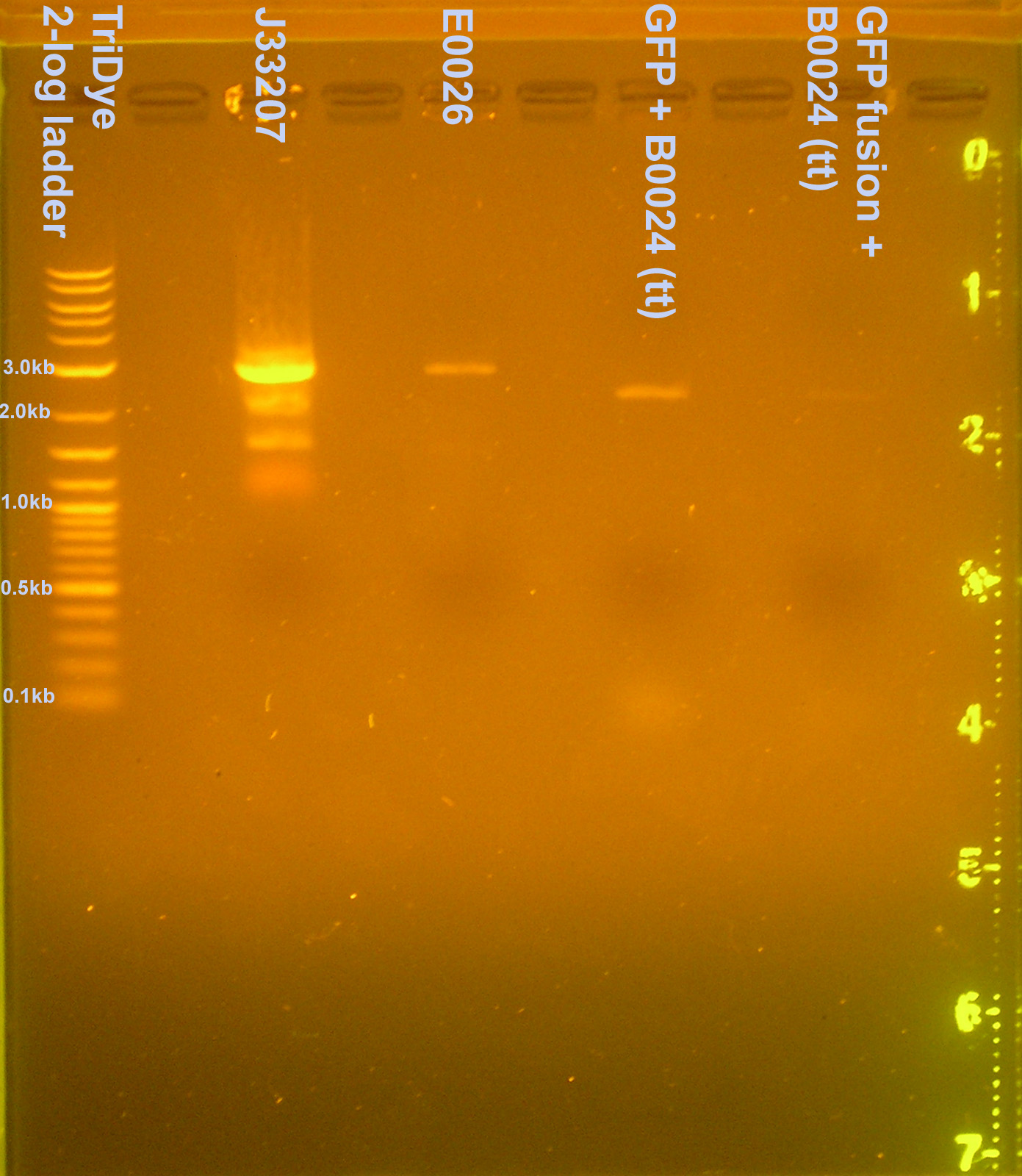
- Digested today's plasmid preps and E0026 and J33207 with EcoRI. Ran on an EtBr stained 1.2% gel to verify. (E0026 and J33207 may have been mixed up as we realized when sequencing returned CFP as the result for the lac part.)
- The newly labeled E0026 (plasmid+part=2841bp) and J33207 (2679bp) plasmid preps are okay but what are the four bands? Linear, ?, ?, supercoiled.
- GFP+B0034 (2874bp) and GFPf+B0034 (2872bp) are too small on the gel. GFP(f) didn't go in? Bands look ~2100bp.
Drylab Work
Sequencing Analysis
- Grace
- All sequence reads are good (forward and/or reverse is of good enough quality to be a significant match against the original sequence) except for BB-pRL1383a. Need to reconstruct BB-pRL1383a because SpeI is the only BioBrick site identified and the insert is not that of BBa_B0034 (Could not ID what it was; blast search returned no results).
Updating Wiki Experiments
- Margaret
- I annotated some pictures and posted some finished experiments plus the plans for Construction of pRL1383a.
Plans for making the pRL1383a construct an inducible high copy number
Margaret
- Started by annotating pSB2K3 on Vector NTI
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"