Team:UNIPV-Pavia/Notebook/Week12
From 2008.igem.org
Notebook
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
|---|---|---|---|---|---|---|
| Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 |
| Week 15 | Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | Week 21 |
| Week 22 | Week 23 | Week 24 |
Week 11: 08/4/08 - 08/7/08
08/4/08
- Plasmid digestion for:
| J23100-B0030-C0040-B1006 (E-S) | R0040 (E-X) |
- Gel run/cut/gel extraction.
- Ligation: J23100-B0030-C0040-B1006-R0040. We incubated ligation at 16°C overnight.
- We had 5 plates to screen with colony PCR:
- B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006 (that we call "a")
- B0030-C0061-B1006-R0062-B0030-E0040-B1006 (that we call "b")
- B0030-C0078-B1006-R0079-B0030-E0040-B1006 (that we call "c")
- B0030-C0061-B0030-C0079-B1006-R0079-B0030-E0040-B1006 (that we call "d")
- J23100-B0030-C0012-B1006-R0010
- Last week J23100-B0030-C0012-B1006-R0010 colony PCR gave a bad result. For this reason, we decided to perform colony PCR only for:
- B0030-C0061-B1006-R0062-B0030-E0040-B1006 (7 colonies)
- J23100-B0030-C0012-B1006-R0010 (6 colonies)
- Gel result:
- B0030-C0061-B1006-R0062-B0030-E0040-B1006 (1st colony, but it was not pure. We decided to prepare single colonies plate for it)
- J23100-B0030-C0012-B1006-R0010 (2nd colony)
08/4/08
- We transformed J23100-B0030-C0040-B1006-R0040 overnight ligation. We plated transformed bacteria and incubated plate at 37°C overnight.
- We infected 9 ml of LB + Amp with 30 µl of C0062, Lig.12, Lig.22, Lig.30, Lig.27(2nd col), B0030-C0061-B1006-R0062-B0030-E0040-B1006 (1st col) (see "Parts" section for our nomenclature).
- Single colonies plates for:
- B0030-C0061-B1006-R0062-B0030-E0040-B1006 ("Lig.b")
- B0030-C0078-B1006-R0079-B0030-E0040-B1006 ("Lig.c")
- B0030-C0061-B0030-C0079-B1006-R0079-B0030-E0040-B1006 ("Lig.d")
08/5/08
- Glycerol stocks/miniprep for C0062, Lig.12, Lig.22, Lig.30, Lig.27(2nd col), B0030-C0061-B1006-R0062-B0030-E0040-B1006(1).
- We sent C0062, Lig.12, Lig.22 and Lig.30 purified plasmids to Primm for sequencing: all these parts contain BBa_C0062.
- We transformed/plated J23100-B0030-C0040-B1006-R0040 overnight ligation.
- Colony PCR for a (7 colonies), Lig.b(single colonies)(6 colonies), Lig.c(single colonies)(6 colonies), Lig.d(single colonies)(6 colonies).
- Gel results were not so clear: the length of some fragments was not expected and there were some contaminants. Maybe those parts were too long for our PCR reaction. We decided to grow 9 ml cultures for some of those colonies, to extract plasmids, to cut them and to check their length in a new run. We chose:
- B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006 (1, 4, 6, 7)
- B0030-C0061-B1006-R0062-B0030-E0040-B1006 (no colony was chosen: we already had them and this run didn't show any 100% pure colony)
- B0030-C0078-B1006-R0079-B0030-E0040-B1006 (5)
- B0030-C0061-B0030-C0079-B1006-R0079-B0030-E0040-B1006 (2)
08/6/08
- Single colonies plate for J23100-B0030-C0040-B1006-R0040, because where were too many bacteria on its plate.
- Glycerol stocks/miniprep for:
- B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(1)
- B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(4)
- B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(6)
- B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(7)
- B0030-C0078-B1006-R0079-B0030-E0040-B1006(5)
- B0030-C0061-B0030-C0079-B1006-R0079-B0030-E0040-B1006(2)
- We cut these 6 plasmids and Lig.b in (E-P) (5 µl of DNA in a final reaction volume of 20 µl).
- Run for digested plasmids.
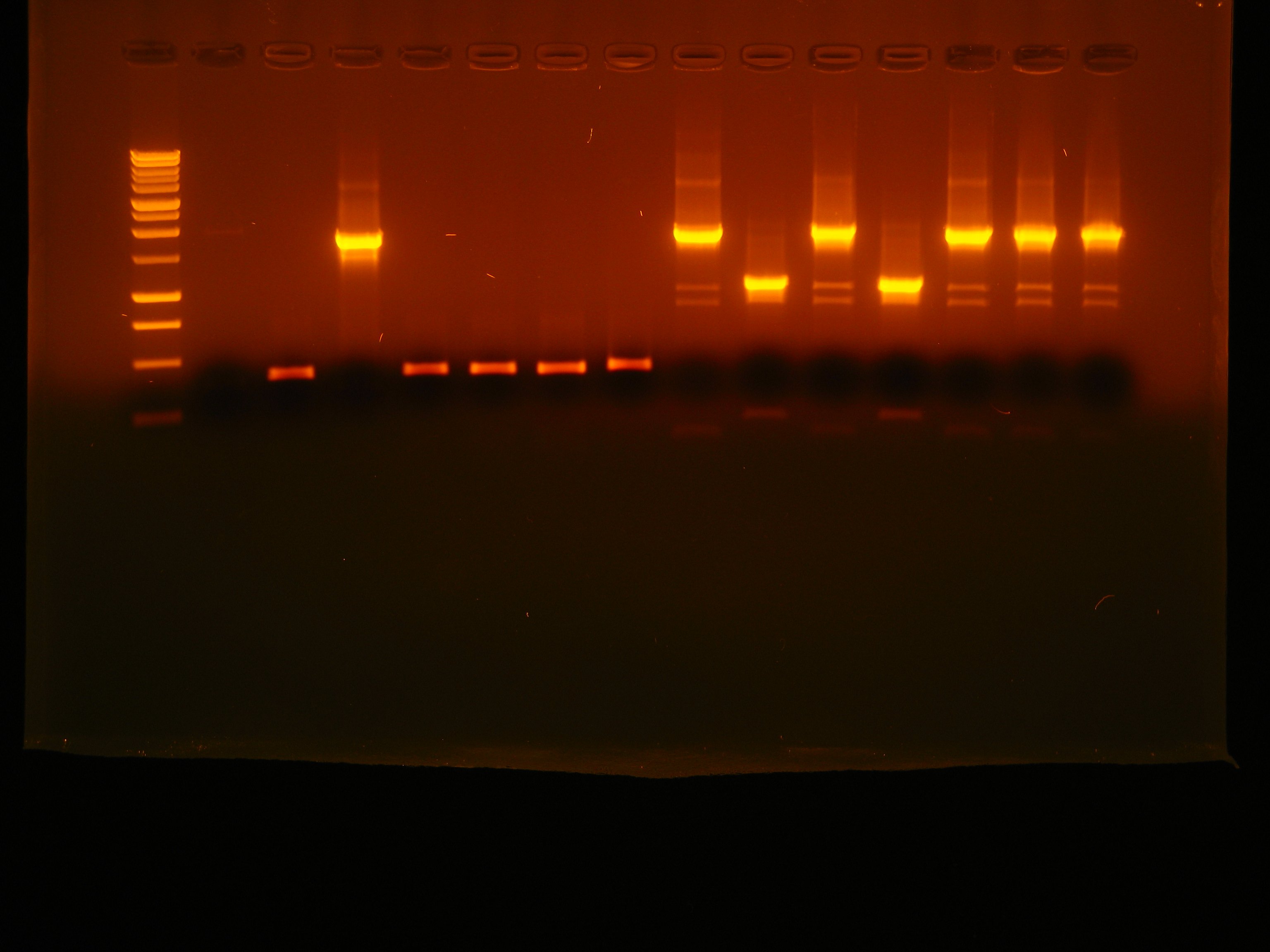
- Gel results:
- (Lig.a) B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(1) OK
- (Lig.a) B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(4) False positive
- (Lig.a) B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(6) OK
- (Lig.a) B0030-C0051-B0030-C0079-B1006-R0051-B0030-C0062-B1006-R0062-B0030-E1010-B1006(7) False positive
- (Lig.b) B0030-C0061-B1006-R0062-B0030-E0040-B1006(1) OK
- (Lig.c) B0030-C0078-B1006-R0079-B0030-E0040-B1006(5) OK
- (Lig.d) B0030-C0061-B0030-C0079-B1006-R0079-B0030-E0040-B1006(2) OK
- We 9 ml of LB + Amp with 30 ul of Lig.b(1) and Lig.c(5) to perform tests.
08/7/08
- Qualitative fluorescence tests for Lig.b and Lig.c. Results are shown in The Project section (Experiments).
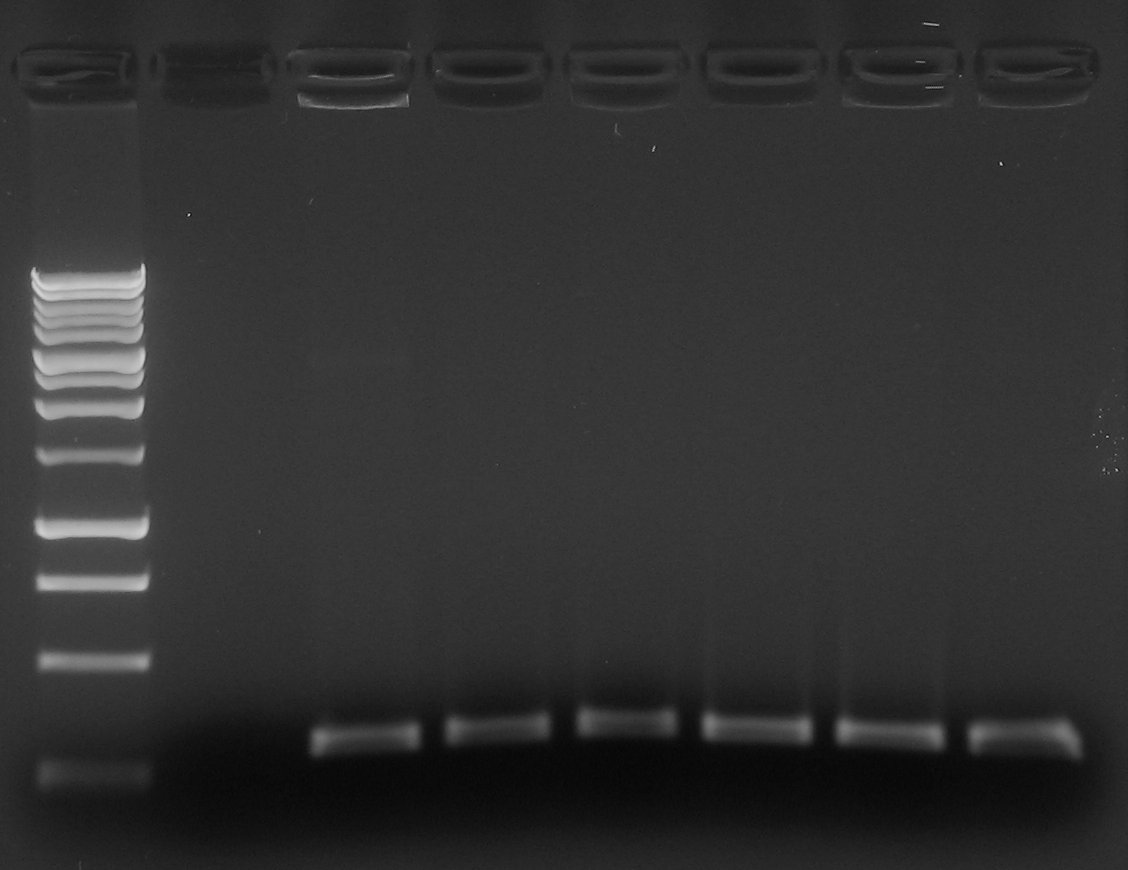
- Colony PCR for J23100-B0030-C0040-B1006-R0040 single colonies plate (screening on 6 colonies).
- Gel results: all screened colonies were negative...
 "
"