Team:Freiburg Cloning Strategy
From 2008.igem.org
| (83 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
<div style="font-size:18pt;"> | <div style="font-size:18pt;"> | ||
<font face="Arial Rounded MT Bold" style="color:#010369">_cloning strategy</font></div> | <font face="Arial Rounded MT Bold" style="color:#010369">_cloning strategy</font></div> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | <h2>Introduction</h2> | ||
| + | |||
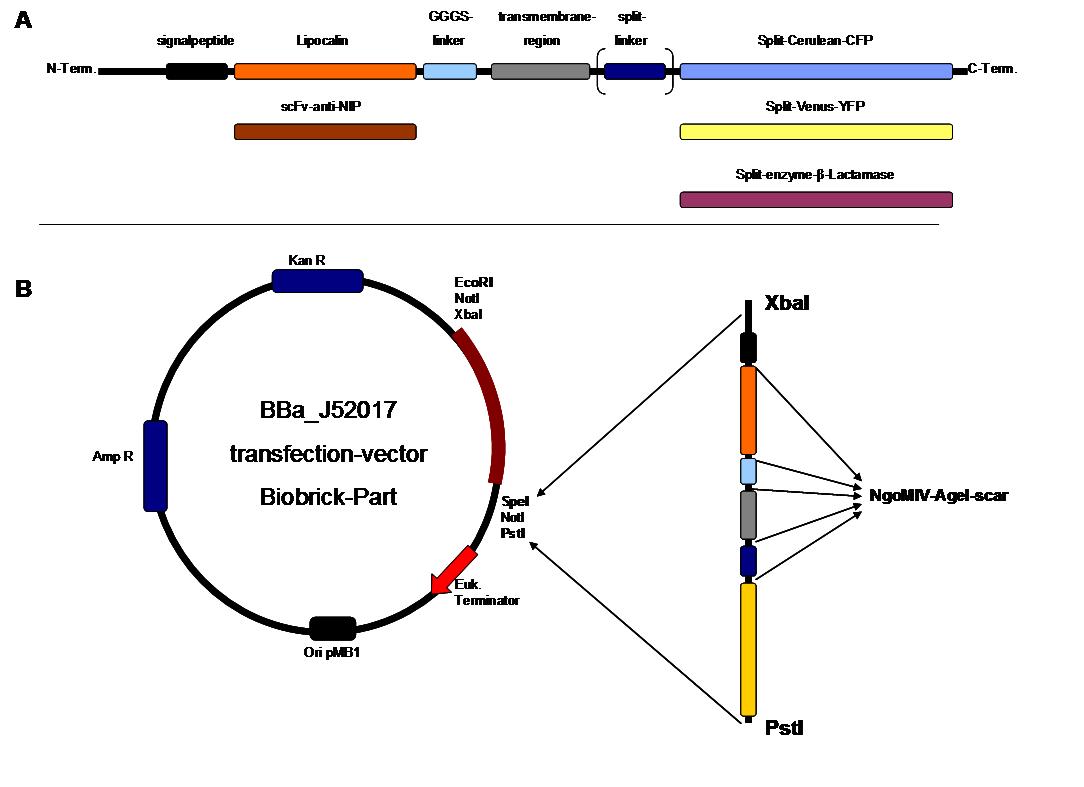
| + | To form different types of synthetic receptor constructs a modular building set was used. We designed the genes of two parts coding for extracellular binding proteins (anti NIP scFv, anti-Flu Lipocalin) and four different components of an intracellular signal transduction reporter protein (Cerulean CFP, Venus YFP, β-Lactamase, and Luciferase). These reporters were designed as split-proteins to achieve activaton only when two receptors come together and form a cluster. With this strategy, a signal is only given by two binding-molecules that are next to each other as it is realized in the DNA-origami structure or the NIP and Fluorescein-coupled BSA. <br> | ||
| + | The whole receptor construct consists out of (i) a signal peptide, (ii) an extracellular receptor domain, (iii) a GGGS-linker followed by (iv) a transmembrane region and (v) an intracellular split-reporter-protein. The signal peptide sequence equal to that of human EGF-receptor erbb1 ensures the transport of the protein construct to the cytoplasma membrane. A GGGS-linker is necessary to create a distance between membrane and the recognition-site to tower cell surface structures as glycoproteins and glycolipids. The sequence of the transmembrane region is taken from the EGF-receptor erbb1 as well. The C-terminal split-parts of Venus-YFP and Cerulean CFP were not directly fused to the transmembrane region. To get a little bit more flexibility in binding to the N-terminal part, a split fluorophor-linker was assembled in between.<br><br> | ||
| + | |||
| + | Almost all parts were ordered by gene synthesis in a pMA-vector system that is adapted for cloning BioBrick parts. The constructs were designed according to BioBrick 3.0 standard with the modification for fusion-proteins proposed by the iGEM Freiburg Team 2007 ([https://2007.igem.org/Freiburg07/report_fusion_parts FreiGEM07_report_fusion_part]). Keeping the standard iGEM prefix and suffix, the restriction sites NgoMIV and AgeI were added to create functional fusions without stop-codons. <br> | ||
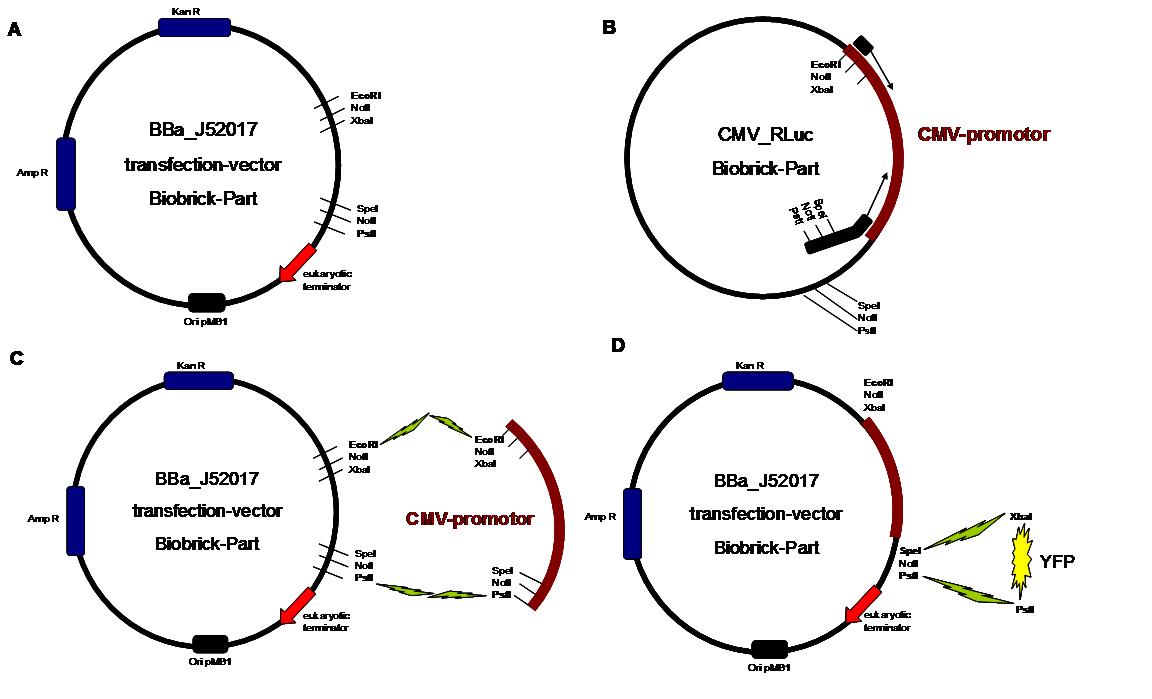
| + | All possible variations of the receptor constructs were fused together in the pMA-vector and afterwards inserted into a transfection vector. The transfection vector is a BioBrick 2006 part from the Ljubljana, Slovenia team ([http://partsregistry.org/Part:BBa_J52017 BBa_J52017]) and includes a kanamycin and ampicillin resistance cassette. There is a pMB1 Ori and the multiple cloning site consist of the BioBrick Prefix and Suffix restriction enzymes (Prefix: EcoRI, NotI, XbaI/ Suffix: SpeI, NotI, PstI) followed by an eukaryotic terminator sequence. To achieve a strong expression, a constitutive promoter construct was needed. Because of this, the Cytomegalovirus [CMV] promotor was amplified by PCR using the BioBrick part [http://partsregistry.org/Part:BBa_J52038 BBa_J52038 ]from the 2006 Ljubljana, Slovenia team as well and primers mentioned below. The forward primer bound in the iGEM-prefix, while the reverse primer bound at the end of the CMV-coding region. The reverse primer also contained an overhang to get the iGEM-suffix directly to the end of the promoter sequence which later allowed the cloning into the transfection vector. The CMV promoter was cloned into the vector by using the EcoRI and PstI restriction sites. To test the expression activity of the vector with the CMV promoter, the gene of the yellow fluorescent protein [YFP] was cloned downstream of the promoter region by using XbaI, PstI restriction enzymes for the YFP insert and SpeI, PstI for the vector to open the Biobrick suffix. The transfection of the resulting plasmid into 293T cells shows full functionality.<br> | ||
| + | An overview about the cloning is given in table 1<br> | ||
<br><br> | <br><br> | ||
| + | [[Image:Freiburg2008_TV_CMVRluc.jpg|700px]] | ||
| + | <br> | ||
| + | <span style="font-weight: bold;">Figure 1_cloning strategy</span> | ||
| + | . <span style="font-weight: bold;">A</span> | ||
| + | <small>shows the Biobrick part BBa_J52017. The transfection vector | ||
| + | for eukaryotic cell systems has an ampicillin and a kanamycin | ||
| + | resistance cassette. The multiple cloning site contains the Biobrick | ||
| + | standard restriction sites EcoRI, NotI, XbaI, SpeI, NotI, PstI followed | ||
| + | by a eukaryotic terminator sequence. <big><span | ||
| + | style="font-weight: bold;">B</span></big> The | ||
| + | CMV promotor fragment was obtained by PCR from the Biobrick BBa-J52038 | ||
| + | template. <big><span style="font-weight: bold;">C</span></big> | ||
| + | The PCR product was cloned into the transfection vector by EcoRI and | ||
| + | PstI to get a final eukaryotic transfection system. <big><span | ||
| + | style="font-weight: bold;">D</span></big> To | ||
| + | test the efficiency of expression, a gene fragment coding for the yellow | ||
| + | fluorescent protein was cloned into the vector downstream of the CMV Promotor</small>. | ||
| + | |||
| + | <br><br> | ||
| + | [[Image:Freiburg2008_Konstrukte.jpg|700px]] | ||
| + | <br> | ||
| + | <span style="font-weight: bold;">Figure 2_cloning strategy</span> | ||
| + | . <span style="font-weight: bold;">A</span> | ||
| + | <small>figure 2 A gives an overview about the cloning constructs. | ||
| + | The N-terminal signal peptide ensures protein transport to the | ||
| + | cytoplasma membrane. Lipocalin and the scFv-anti-NIP are the | ||
| + | extracytoplasmatic parts of the construct to mediate signal transduction | ||
| + | into the cell. The GGGS-Linker keeps a distance to the | ||
| + | transmembrane region to overcome surface structures of the cell and to | ||
| + | avoid a total inflexibility. The split fluorophor linker is only necessary | ||
| + | for the C-terminal split parts of Cerulean-CFP and | ||
| + | Venus-YFP. The split enzymes β-Lactamase and Luciferase | ||
| + | and the split-fluorophors CFP and YFP are the cytoplasmatic parts of | ||
| + | the constructs. If there is a clustering of this synthetic | ||
| + | receptor-system caused by the corresponding binding parts of Lipocalin | ||
| + | and scFv-anti-NIP, the split parts come together to create a functional | ||
| + | protein, which allows detection. </small><br> | ||
| + | <span style="font-weight: bold;">B</span> <small>The | ||
| + | different constructions described in figure 2.A were cloned into the | ||
| + | transfection vector system by using the restriction sites XbaI and PstI | ||
| + | to ensure a functional ATG-start codon which is part of the XbaI | ||
| + | recognition-sequence in the iGEM-prefix.</small> | ||
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | <p class="MsoNormal"><b style=""><span | ||
| + | style="" lang="EN-GB">Table1_cloning strategy </span></b><small><span | ||
| + | style="" lang="EN-GB">overview about<b style=""> | ||
| + | </b>the cloning steps to create the different types of synthetic | ||
| + | receptors. To get further information about the composite parts see </span></small></p> | ||
| + | <p class="MsoNormal"><span style="" lang="EN-GB"><small>https://2008.igem.org/Team:Freiburg/Parts</small> | ||
| + | <br><br> | ||
| + | |||
<table class="MsoTableGrid" | <table class="MsoTableGrid" | ||
style="border: medium none ; border-collapse: collapse;" border="1" | style="border: medium none ; border-collapse: collapse;" border="1" | ||
| Line 922: | Line 986: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| + | <br> | ||
| - | [ | + | <h2>Methods</h2> |
| + | |||
| + | |||
| + | The cloning was started with a preparative digestion of the DNA-Plasmids. To clone fusion parts the vector constructs were digested with AgeI and PstI to open the Biobrick suffix. The inserts were digested with NgoMIV and PstI. For cloning into the transfection-vector the enzymes SpeI and PstI were used for vector and XbaI, PstI for insert to keep up the ATG-start codon in the XbaI restriction site of the biobrick suffix. All restriction-enzymes were ordered from New England Biolabs. After digestion the DNA-fragments were separated on a 1% agarose gel. The DNA-band of interest was isolated and purified with the QIAGEN QIAquick Gel Extraction Kit. For the ligation a 3 molar excess of the insert was put together with the vector-fragment and ligated with a Quick ligase (New England Biolabs). After half an our at room temperature the DNA was transformed to chemical competent E.coli strain XL1 cells, plated on 2YT-agar-plates and incubated at 37°C over night. After picking clones and growing in 5ml LB-medium, the plasmid DNA was isolated by QIAGEN QIAprep Spin Miniprep Kit. A test digestion was prepared with about 0,5µg Plasmid DNA and NotI restriction enzyme to isolate the fusion-protein from the vector and to control if the expected bands were obtained. After a positive result the clones were sent to GATC-Biotech for sequencing. | ||
| + | The GGGS-Linker was produced by Klenow -fill-in-PCR. Two primers were designed align to each other at 60°C and filled to a complete double-strand by Klenow Polymerase fragment.<br> | ||
| + | <br> | ||
| + | <h4>Digestion Protocol</h4> | ||
| + | - about 2µg Plasmid-Prep in 20µl <br> | ||
| + | - 2 µl NEB Buffer 10x<br> | ||
| + | - 1 µl NEB enzyme 1 (NgoMIV, AgeI, XbaI, EcoRI)<br> | ||
| + | - 1 µl NEB enzyme 2 (PstI, SpeI)<br> | ||
| + | - 0.2µl BSA 100x<br> | ||
| + | <br> | ||
| + | <h4>Ligation</h4> | ||
| + | - 10µl volume of vector and insert DNA (about 50ng vector-DNA)<br> | ||
| + | - 1 µl DNA Quick Ligase (New England Biolabs)<br> | ||
| + | - 10 µl Quick Ligase Buffer<br> | ||
| + | <br> | ||
| + | <h4>Analytic digestion</h4> | ||
| + | - about 0.5 µg Plasmid-DNA in 5µl<br> | ||
| + | - 5µl H2O<br> | ||
| + | - 0.5 µl NotI<br> | ||
| + | - 1µl NEB-Buffer<br> | ||
| + | - 0.1 µl BSA<br> | ||
| + | <br> | ||
| + | <h4>Transformation</h4> | ||
| + | - Competent cells (100µl) werde defrosted on ice<br> | ||
| + | - 10µl of the ligation was added<br> | ||
| + | - DNA and cells werde mixed softly <br> | ||
| + | - Incubation on ice for 20-30 min<br> | ||
| + | - Heat shock at 42°C for 40 sek <br> | ||
| + | - cells were cooled down on ice for 5-10 min<br> | ||
| + | - 900µl sterile 2YT Medium was added<br> | ||
| + | - Incubation at 37°C for 60-70 min (shaker)<br> | ||
| + | - cells werde plated on 2YT-agar-plates with antibiotics<br> | ||
| + | <br> | ||
| + | <h4>Klenow fill in reaction</h4> | ||
| + | - 25pmol forward primer<br> | ||
| + | - 25pmol reverse primer<br> | ||
| + | - 0.5 µl Klenow-fragment without exonuclease activity (Fermentas)<br> | ||
| + | - 2µl Klenow Buffer<br> | ||
| + | - 1µl dNTPs<br> | ||
| + | - Add H2O to a volume of 20µl<br> | ||
| + | program: 94°C for 3min, cool down to 37°C, adition of klenow enzyme, 37°C for 1 hour | ||
| + | |||
| + | |||
| + | <h2>Results</h2> | ||
| + | |||
| + | All composite parts were succesfully cloned and controlled by sequencing and transfection as well ([https://2008.igem.org/Team:Freiburg_Transfection_and_Synthetic_Receptor Freiburg Transfection and Synthetic Receptor]) | ||
| + | |||
| + | <h2>Biobrick fusion protein</h2> | ||
| + | |||
| + | The present BioBrick prefix and suffix rules are not compatible with modular protein design. Thus as in 2007, we proposed an extension of the present standard for fusion proteins in which two restriction sites are added in frame adjacent to the coding sequence. The basic parts and as well all composite parts follow this strategy. | ||
| + | To get further information see [https://2007.igem.org/Freiburg07/report_fusion_parts FreiGEM07_report_fusion_part]<br> | ||
| + | |||
| + | |||
| + | <h2>Cloned parts</h2> | ||
| - | [[ | + | The cloning parts scFv-anti-NIP, lipocalin-FluA, Split-Venus-N-YFP, Split-Venus-C-YFP, Cerulean-N-CFP and Cerulean-C-CFP were obtained by genesynthesis and ordered by the GENEART company. The β-Lactamase fragments were adopted from genesynthesis GENEART order in 2007. The erbb1-transmembrane, Split-Fluorophor-Linker and the erbb1-signalpeptide were synthesized by ATG:biosynthetics. The CMV promoter cloning part was produced by PCR with the template J52038 using the CMV_RLuc_Forward-primer and CMV_RLuc_Reverse-primer. To synthesize the GGGS-linker a Klenow-fill-in-reaction was performed by using Klenow-fragment without exonuclease activity and GGGS-forward and reverse primer.<br> |
| + | <br> | ||
| + | CMV-Promotor-PCR [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157040 BBa_K157040]<br> | ||
| + | CMV_RLuc_Forward-primer: <span style="font-family: monospace;">TCGCTAAGGA TGATTTCTGG AA</span><br> | ||
| + | CMV_RLuc_Reverse-primer: <span style="font-family: monospace;">AACGATCCTG CAGCGGCCGC TACTAGTAAT TTCGATAAGC CAGTAAGCAG</span><br> | ||
| + | <br> | ||
| + | GGGS-Linker (primer-fill-in) <br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">GGGSGGGSGG GSGGG</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">GGAGGAGGTG GTTCAGGTGG TGGTGGTAGT GGAGGAGGAG GATCC</span><br> | ||
| + | <br> | ||
| + | GGGS_Forward-primer: <span style="font-family: monospace;">ATTATATTGA ATTCGCGGCC GCTTCTAGAt gGCCGGCGGA GGAGGTGGTT CAGGTGGTGG TGGTAGTGGA</span><br> | ||
| + | GGGS_Reverse-primer: | ||
| + | <span style="font-family: monospace;">TTATATTTCT GCAGCGGCCG CTACTAGTAT TAACCGGTGG ATCCTCCTCC TCCACTACCA CCACCACCT</span><br> | ||
| + | <br> | ||
| + | GGGS-linker (pMA) [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157013 BBa_K157013]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">GGGSGGGSGG GSGGG</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">GGTGGAGGAG GTTCTGGAGG CGGTGGAAGT GGTGGCGGAG GTAGC</span><br> | ||
| + | <br> | ||
| + | Erbb1-transmembraneregion [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157002 BBa_K157002]<br> | ||
| + | IAA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">ATGMVGALLL LLVVALGIGL FM</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">ATAGCTACCG GAATGGTGGG TGCACTTTTG CTCCTTTTGG TCGTTGCCCT GGGGATAGGA CTCTTTATG</span><br> | ||
| + | <br> | ||
| + | Signalpeptide [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157001 BBa_K157001]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">MRPSGTAGAA LLALLAALCP ASRA</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">ATGAGACCAT CTGGTACTGC TGGAGCCGCA TTGCTGGCAC TTTTGGCTGC GCTGTGCCCT GCAAGCAGAG CA</span><br> | ||
| + | <br> | ||
| + | Split-Fluo-Linker [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157009 BBa_K157009]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">RPACKIPNDL KQKVMNH</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">CGACCAGCCT GTAAGATTCC AAATGACCTG AAGCAGAAAG TTATGAATCA C</span><br> | ||
| + | <br> | ||
| + | Sc-Fv anti NIP [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157003 BBa_K157003]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">QVQLQQPGAE LVKPGASVKL SCKASGYTFT SYWMHWVKQR PGRGLEWIGR IDPNSGGTKY NEKFKSKATL TVDKPSSTAY MQLSSLTSED SAVYYCARYD YYGGSYFDYW GQGTTVTVSS GGGGSGGGGS GGGGSQAVVT QESALTTSPG ETVTLTCRSS TGAVTTSNYA NWVQEKPDHL FTGLIGGTNN RAPGVPARFS GSLIGDKAAL TITGAQTEDE AIYFCALWYS NHWVFGGGTK LTVLG</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">CAGGTGCAGC TCCAGCAGCC CGGAGCCGAA CTGGTGAAGC CAGGCGCCAG CGTGAAGCTG TCCTGCAAGG CCAGCGGCTA CACCTTCACC AGCTACTGGA TGCACTGGGT GAAACAGAGG CCCGGCAGAG GCCTGGAATG GATCGGCCGG ATCGACCCCA ACAGCGGCGG CACCAAGTAC AACGAGAAGT TCAAGAGCAA GGCCACCCTG ACCGTGGACA AGCCCAGCAG CACCGCCTAC ATGCAGCTGT CCAGCCTGAC CAGCGAGGAC AGCGCCGTGT ACTACTGCGC CAGATACGAC TACTACGGCG GCAGCTACTT CGACTACTGG GGCCAGGGCA CCACCGTGAC CGTGTCCTCT GGGGGAGGGG GCTCAGGAGG AGGAGGAAGC GGGGGAGGGG GCAGCCAGGC CGTGGTGACC CAGGAAAGCG CCCTGACCAC TCCCCTGGCG GACAGTGACC TGACCTGCCG TCCTCTACAG GCGCCGTGAC CACAAGCAAC TACGCCAACT GGGTGCAGGA AAAGCCCGAC CACCTGTTCA CCGGCCTGAT CGGCGGCACA AACAACAGAG CCCCTGGCGT GCCCGCTAGA TTCAGCGGCA GCCTGATCGG GGACAAGGCC GCCCTGACAA TCACAGGCGC CCAGACCGAG GACGAGGCCA TCTACTTTTG CGCCCTGTGG TACAGCAACC ACTGGGTGTT CGGCGGAGGG ACCAAGCTGA CCGTGCTGGG C</span><br> | ||
| + | <br> | ||
| + | Split-Venus-N-YFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157008 BBa_K157008]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">MVSKGEELFT GVVPILVELD GDVNGHKFSV SGEGEGDATY GKLTLKLICT TGKLPVPWPT LVTTLYLQCF ARYPDHMKQH DFFKSAMPEG YVQERTIFFK DDGNYKTRAE VKFEGDTLVN RIELKGIDFK EDGNILGHKL EYNYNSHNVY ITADKQ</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">ATGGTGTCCA AGGGCGAGGA ACTGTTCACC GGCGTGGTGC CCATCCTGGT GGAGCTGGAC GGCGACGTGA ACGGCCACAA GTTCAGCGTG TCCGGCGAGG GCGAAGGCGA CGCCACCTAC GGCAAGCTGA CCCTGAAGCT GATCTGCACC ACCGGCAAGC TGCCCGTGCC CTGGCCCACC CTGGTGACCA CCCTGTACCT CCAGTGCTTC GCCAGATACC CCGACCACAT GAAGCAGCAC GATTTCTTCA AGAGCGCCAT GCCCGAGGGC TACGTGCAGG AACGGACCAT CTTCTTCAAG GACGACGGCA ACTACAAGAC CAGAGCCGAA GTGAAGTTCG AGGGCGACAC ACTGGTGAAC CGGATCGAGC TGAAGGGCAT CGACTTCAAA GAGGACGGCA ATATCCTGGG CCACAAGCTG GAATACAACT ACAACAGCCA CAACGTGTAC ATCACCGCCG ACAAGCAG</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | <br> | ||
| + | Split-Venus-C-YFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157007 BBa_K157007]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">KNGIKANFKI RHNIEDGGVQ LADHYQQNTP IGDGPVLLPD NHYLSYQSKL SKDPNEKRDH MVLLEFVTAA GITHGMDELY K</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">AAGAACGGCA TCAAGGCCAA CTTCAAGATC CGGCACAACA TCGAGGACGG CGGCGTGCAG CTGGCCGACC ACTACCAGCA GAACACCCCC ATCGGCGACG GCCCCGTGCT GCTGCCCGAC AACCACTACC TGAGCTACCA GAGCAAGCTG TCCAAGGACC CCAACGAGAA GCGGGACCAC ATGGTGCTGC TGGAATTTGT GACAGCCGCC GGAATCACCC ACGGCATGGA CGAGCTGTAC AAG</span><br> | ||
| + | <br> | ||
| + | Cerulean-N-CFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157006 BBa_K157006]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">MSKGEELFTG VVPILVELDG DVNGHKFSVS GEGEGDATYG KLTLKFICTT GKLPVPWPTL VTTLGVQCFA RYPDHMKRHD FFKSAMPEGY VQERTIFFKD DGNYKTRAEV KFEGDTLVNR IELKGIDFKE DGNILGHKLE YNAISDNVYI TADKQ</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">ATGAGCAAGG GCGAGGAACT GTTCACCGGC GTGGTGCCCA TCCTGGTGGA GCTGGACGGC GACGTGAACG GCCACAAGTT CAGCGTGTCC GGCGAGGGCG AAGGCGACGC CACCTACGGC AAGCTGACCC TGAAGTTCAT CTGCACCACC GGCAAGCTGC CCGTGCCCTG GCCCACCCTG GTGACCACCC TGGGCGTGCA GTGCTTCGCC AGATACCCCG ACCACATGAA GCGGCACGAT TTCTTCAAGA GCGCCATGCC CGAGGGCTAC GTGCAGGAAC GGACCATCTT CTTCAAGGAC GACGGCAACT ACAAGACCAG AGCCGAAGTG AAGTTCGAGG GCGACACACT GGTGAACCGG ATCGAGCTGA AGGGCATCGA CTTCAAAGAG GACGGCAATA TCCTGGGCCA CAAGCTGGAA TACAACGCCA TCAGCGACAA CGTGTACATC ACCGCCGACA GCAG</span><br> | ||
| + | <br> | ||
| + | Cerulean-C-CFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157005 BBa_K157005]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">KNGIKANFKI RHNIEDGSVQ LADHYQQNTP IGDGPVLLPD NHYLSTQSKL SKDPNEKRDH</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">MVLLEFVTAA GITLGMDELY KAAGAACGGC ATCAAGGCCA ACTTCAAGAT CCGGCACAAC ATCGAGGATG GCAGCGTGCA GCTGGCCGAT CACTACCAGC AGAACACCCC CATCGGCGAC GGCCCCGTGC TGCTGCCCGA CAACCACTAC CTGAGCACCC AGAGCAAGCT GTCCAAGGAC CCCAACGAGA AGCGGGACCA CATGGTGCTG CTGGAATTTG TGACAGCCGC CGGAATCACC CTGGGCATGG ACGAGCTGTA CAAG</span><br> | ||
| + | <br> | ||
| + | Split beta-Lactamase 1/2 [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157018 BBa_K157018]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">MAGIATGMVG ALLLLLVVAL GIGLFMTGHP ETLAKVKDAE DQLGARVGYI ELDLNSGKIL ESFRPEERFP MMSTFKVLLC GAVLSRIDAGQ EQLGRRIHYS QNDLVEYSPV TEKHLTDGM TVGELCSAAI TMSDNTAANL LLTTIGGPKE LTAFLRNMGDH VTRLDRWEPE LNEAIPNDE RDTTTPVAMAT TLRKLLTGEL LGTG*</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">ATGGCCGGCA TAGCTACCGG AATGGTGGGT GCACTTTTGC TCCTTTTGGT CGTTGCCCTG | ||
| + | GGGATAGGAC TCTTTATGAC CGGCCACCCG GAAACCCTGG CCAAAGTGAA AGATGCGGAA GATCAGCTGG GTGCGCGTGT GGGCTATATT GAACTGGATC TGAACAGCGG CAAAATTCTG GAATCTTTTC GTCCGGAAGA ACGTTTTCCG ATGATGAGCA CCTTTAAAGT GCTGCTGTGC | ||
| + | GGTGCGGTTC TGAGCCGTAT TGATGCGGGC CAGGAACAGC TGGGCCGTCG TATTCATTAT AGCCAGAACG ATCTGGTGGA ATATAGCCCG GTGACCGAAA AACATCTGAC CGATGGCATG ACCGTGGGCG AACTGTGCAG CGCGGCGATT ACCATGAGCG ATAACACCGC GGCGAACCTG CTGCTGACCA CCATTGGCGG TCCGAAAGAA CTGACCGCGT TTCTGCGTAA CATGGGCGAT CATGTGACCC GTCTGGATCG TTGGGAACCG GAACTGAACG AAGCGATTCC GAACGATGAA | ||
| + | CGTGATACCA CCACCCCGGT GGCCATGGCG ACCACCCTGC GTAAACTGCT GACCGGCGAA CTGCTGGGCA CCGGTTAA</span><br> | ||
| + | <br> | ||
| + | Split beta-Lactamase 2/2 [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157019 BBa_K157019]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">MAGIATGMVG ALLLLLVVAL GIGLFMTGGT PASRQQLMDW MKADKVAGPL LRSVLPAGWF IADKSGAGER GSRGIIAALG PDGKPSRIVV IYTTGSQATM DELNRQIAEI GASLIKHWTG * | ||
| + | </span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">ATGGCCGGCA TAGCTACCGG AATGGTGGGT GCACTTTTGC TCCTTTTGGT CGTTGCCCTG GGGATAGGAC TCTTTATGAC CGGCGGTACT CCGGCTTCCC GGCAACAATT AATGGACTGG ATGAAAGCGG ATAAAGTTGC AGGACCACTT CTGCGCTCGG TGCTTCCGGC TGGCTGGTTT ATTGCTGATA AATCTGGAGC CGGTGAGCGT GGGTCTCGCG GTATCATTGC AGCACTGGGG CCAGATGGTA AGCCCTCCCG TATCGTAGTT ATCTACACGA CGGGGAGTCA GGCAACTATG GATGAACTGA ATCGTCAGAT CGCTGAGATA GGTGCCTCAC TGATTAAGCA TTGGACCGGT | ||
| + | TAA</span><br> | ||
| + | <br> | ||
| + | Split Luciferase 58 [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157020 BBa_K157020]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">EAIVDIPEIP GFKDLEPMEQ FIAQVDLCVD CTTGCLKGLA NVQCSDLLKK WLPQRCATFA SKIQGQVDKI KGAGGD</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">GAAGCGATTG TGGATATTCC GGAAATTCCG GGCTTTAAAG ATCTGGAACC GATGGAACAG TTTATTGCGC AGGTGGATCT GTGCGTGGAT TGCACCACCG GCTGCCTGAA AGGCCTGGCC AACGTGCAGT GCAGCGATCT GCTGAAAAAA TGGCTGCCGC AGCGTTGCGC GACCTTTGCG AGCAAAATTC AGGGCCAGGT GGATAAAATT AAAGGCGCGG GTGGCGAT</span><br> | ||
| + | <br> | ||
| + | Split Luciferase 57 [http://partsregistry.org/wiki/index.php?title=Part:BBa_K157021 BBa_K157021]<br> | ||
| + | AA sequence:<br> | ||
| + | <span style="font-family: monospace; font-weight: bold;">KPTENNEDFN IVAVASNFAT TDLDADRGKL PGKKLPLEVL KEMEANARKA GCTRGCLICL SHIKCTPKMK KFIPGRCHTY EGDKESAQGG IG</span><br | ||
| + | style="font-family: monospace;"> | ||
| + | gene sequence:<br> | ||
| + | <span style="font-family: monospace;">AAACCGACCG AAAACAACGA AGATTTTAAC ATTGTGGCGG TGGCGAGCAA CTTTGCGACC ACCGATCTGG ATGCGGATCG TGGCAAACTG CCGGGCAAAA AACTGCCGCT GGAAGTGCTG AAAGAAATGG AAGCGAACGC GCGTAAAGCC GGTTGCACCC GTGGCTGCCT GATTTGCCTG AGCCATATTA AATGCACCCC GAAAATGAAA AAATTTATCC CGGGTCGTTG CCATACCTAT GAAGGCGATA AAGAAAGCGC GCAGGGCGGC ATTGGC</span><br> | ||
}} | }} | ||
Latest revision as of 09:01, 16 November 2008
|
_cloning strategy
IntroductionTo form different types of synthetic receptor constructs a modular building set was used. We designed the genes of two parts coding for extracellular binding proteins (anti NIP scFv, anti-Flu Lipocalin) and four different components of an intracellular signal transduction reporter protein (Cerulean CFP, Venus YFP, β-Lactamase, and Luciferase). These reporters were designed as split-proteins to achieve activaton only when two receptors come together and form a cluster. With this strategy, a signal is only given by two binding-molecules that are next to each other as it is realized in the DNA-origami structure or the NIP and Fluorescein-coupled BSA. Almost all parts were ordered by gene synthesis in a pMA-vector system that is adapted for cloning BioBrick parts. The constructs were designed according to BioBrick 3.0 standard with the modification for fusion-proteins proposed by the iGEM Freiburg Team 2007 (FreiGEM07_report_fusion_part). Keeping the standard iGEM prefix and suffix, the restriction sites NgoMIV and AgeI were added to create functional fusions without stop-codons.
Table1_cloning strategy overview about the cloning steps to create the different types of synthetic receptors. To get further information about the composite parts see https://2008.igem.org/Team:Freiburg/Parts
Step 1 Vector digestion: EcoRI + PstI Insert
digestion: EcoRI + PstI BBa-J52017 _CMV-promotor Step
2 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_ SPLIT-Linker C-YFP C-CFP Step
3 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_egfR-Tm _ N-β-Lactamase _ C-β-Lactamase _ SPLIT-Linker_ C-YFP _ N-YFP _ SPLIT-Linker_ C-CFP _ N-CFP _ BB058 (Luciferase) _ BB057 (Luciferase) Step
4 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_SP _scFv-anti-NIP _ Lipocalin Step
5 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_SP_ scFv-anti-NIP and pMA-BBFR-+SP_ Lipocalin _GGGS-linker (produced by Klenow fill in) Step
6 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_SP_ scFv-anti-NIP _ GGGS-Li and pMA-BBFR
_ SP_ Lipocalin _ GGGS-Li _
egfR-Tm _ N-β-Lactamase _
egfR-Tm _ C-β-Lactamase _
egfR-Tm _ SPLIT-Linker_ C-YFP _
egfR-Tm _ N-YFP _
egfR-Tm _ SPLIT-Linker_ C-CFP _
egfR-Tm _ N-CFP _
egfR-Tm _ BB058 (Luciferase) _
egfR-Tm _ BB057 (Luciferase) Step
7 Vector digestion:
SpeI + PstI Insert
digestion: XbaI + PstI BBa-J52017_CMV _SP_ scFv-anti-NIP_GGGS-Li_egfR-Tm_N-β-Lactamase _ SP_ scFv-anti-NI _GGGS-Li_ egfR-Tm_C-β-Lactamase _ SP_ scFv-anti-NIP_GGGS-Li_
egfR-Tm_SPLIT-Linker_C-YFP _ SP_ scFv-anti-NIP_GGGS-Li_ egfR-Tm_N-YFP _ SP_ scFv-anti-NIP_GGGS-Li_
egfR-Tm_SPLIT-Linker_C-CFP _ SP_ scFv-anti-NIP_GGGS-Li_ egfR-Tm_N-CFP _ SP_ scFv-anti-NIP_GGGS-Li _ egfR-Tm_BB058 (Luciferase) _ SP_ scFv-anti-NIP_GGGS-Li _ egfR-Tm_BB057 (Luciferase) _ SP_ Lipocalin _GGGS-Li_ egfR-Tm_N-β-Lactamase _ SP_ Lipocalin _GGGS-Li_ egfR-Tm_C-β-Lactamase _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_SPLIT-Linker_ C-YFP _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_N-YFP _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_SPLIT-Linker_ C-CFP _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_N-CFP _ SP_ Lipocalin _GGGS-Li__ egfR-Tm _ BB058 (Luciferase) _ SP_ Lipocalin _GGGS-Li__ egfR-Tm _ BB057 (Luciferase) - about 2µg Plasmid-Prep in 20µl - 10µl volume of vector and insert DNA (about 50ng vector-DNA) - about 0.5 µg Plasmid-DNA in 5µl - Competent cells (100µl) werde defrosted on ice - 25pmol forward primer All composite parts were succesfully cloned and controlled by sequencing and transfection as well (Freiburg Transfection and Synthetic Receptor)
The present BioBrick prefix and suffix rules are not compatible with modular protein design. Thus as in 2007, we proposed an extension of the present standard for fusion proteins in which two restriction sites are added in frame adjacent to the coding sequence. The basic parts and as well all composite parts follow this strategy.
To get further information see FreiGEM07_report_fusion_part The cloning parts scFv-anti-NIP, lipocalin-FluA, Split-Venus-N-YFP, Split-Venus-C-YFP, Cerulean-N-CFP and Cerulean-C-CFP were obtained by genesynthesis and ordered by the GENEART company. The β-Lactamase fragments were adopted from genesynthesis GENEART order in 2007. The erbb1-transmembrane, Split-Fluorophor-Linker and the erbb1-signalpeptide were synthesized by ATG:biosynthetics. The CMV promoter cloning part was produced by PCR with the template J52038 using the CMV_RLuc_Forward-primer and CMV_RLuc_Reverse-primer. To synthesize the GGGS-linker a Klenow-fill-in-reaction was performed by using Klenow-fragment without exonuclease activity and GGGS-forward and reverse primer. |
 "
"