Team:Heidelberg/Notebook/Sensing Group/Notebook/2ndweek
From 2008.igem.org
(Difference between revisions)
Chenchenzhu (Talk | contribs) (→Friday, 08/15/2008) |
(→Tuesday, 08/12/2008) |
||
| Line 128: | Line 128: | ||
== Tuesday, 08/12/2008 == | == Tuesday, 08/12/2008 == | ||
* Miniprep of correctly sequenced LuxS and Glycerol-stock | * Miniprep of correctly sequenced LuxS and Glycerol-stock | ||
| - | * LuxQ no. 3 checked via digestion with NcoI/BamHI --> negative result | + | * LuxQ no. 3 checked via digestion with NcoI/BamHI (NEBuffer 3 + BSA) --> negative result |
* PCR for LuxQ, LuxQ-1, LuxQ-2, Tar-1, Tar-2 with Taq Mastermix | * PCR for LuxQ, LuxQ-1, LuxQ-2, Tar-1, Tar-2 with Taq Mastermix | ||
** 5min @ 95°C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles) | ** 5min @ 95°C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles) | ||
Revision as of 23:33, 26 October 2008


Contents |
Monday, 08/11/2008
- preparation of LuxS harboring cells O/N culture
Tuesday, 08/12/2008
- Miniprep of correctly sequenced LuxS and Glycerol-stock
- LuxQ no. 3 checked via digestion with NcoI/BamHI (NEBuffer 3 + BSA) --> negative result
- PCR for LuxQ, LuxQ-1, LuxQ-2, Tar-1, Tar-2 with Taq Mastermix
- 5min @ 95°C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles)
- preparation of O/N culture for LuxQ no. 3
Wednesday, 08/13/2008
- PCR-purification of the all PCR-products eluted in 50 µl H2O
- Miniprep of LuxQ no. 3 O/N culture and digestion with xbaI
- Fusion-PCR for Fusion-1 and repetition of PCR for LuxQ-2 and LuxQ
- digestion of pDK48 plasmid and Fusion-1 frament with NcoI/NdeI (0.5 µl) and AgeI (1 µl) in NEBuffer 4
Thursday, 08/14/2008
- Gel for LuxQ, LuxQ2
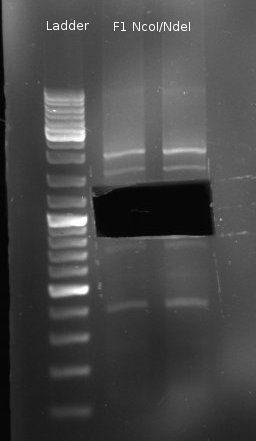
- sequentiell digestion of Fusion-1 with NcoI and NdeI (NEBuffer 3)
- Double digestion of pDK48 with NcoI/NdeI (NEBuffer 4)
Friday, 08/15/2008
- digestion of Fusion-1 with AgeI in NEBuffer 1
- gel extraction of sequentially digested Fusion-1 and pDK48 products
- new digestion of Fusion-1 with NcoI/NdeI and gel extraction, because previous digestion did not yield expected bands (there should be two small bands at 516bp and 392bp)
- Ligation of 5 µl Fusion-1 with 5 µl pDK48 (both digested with NcoI/NdeI )
- Transformation into DH5alpha (5 µl Ligation products)
 "
"