Team:Brown/Project/Testing
From 2008.igem.org
NeilParikh (Talk | contribs) |
NeilParikh (Talk | contribs) (→Design V1) |
||
| Line 7: | Line 7: | ||
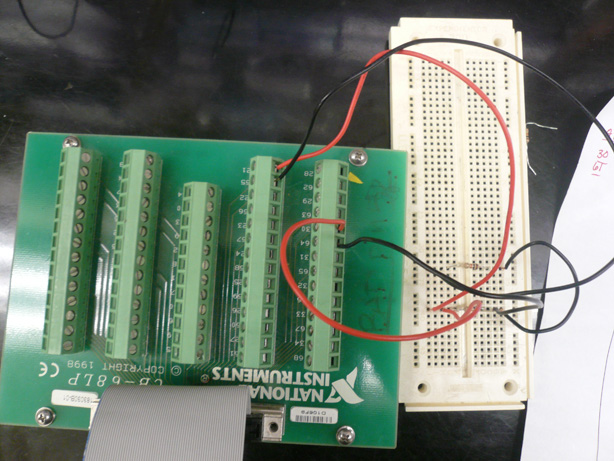
Team Toxipop started off the summer focusing on measuring changes in resistance. Much of our summer work was devoted to the construction of an electrical circuit and measuring apparatus unique to our purpose. Many additions were made to the apparatus each week. Below, you will find information about the different "versions" of the apparatus including final information about our switch to Conductivity. | Team Toxipop started off the summer focusing on measuring changes in resistance. Much of our summer work was devoted to the construction of an electrical circuit and measuring apparatus unique to our purpose. Many additions were made to the apparatus each week. Below, you will find information about the different "versions" of the apparatus including final information about our switch to Conductivity. | ||
==Design V1== | ==Design V1== | ||
| + | [[Image:BrownVoltmeter.jpg|right|thumb|350px|Voltmeter]] | ||
| + | |||
==Design V2== | ==Design V2== | ||
Revision as of 04:15, 29 October 2008
|
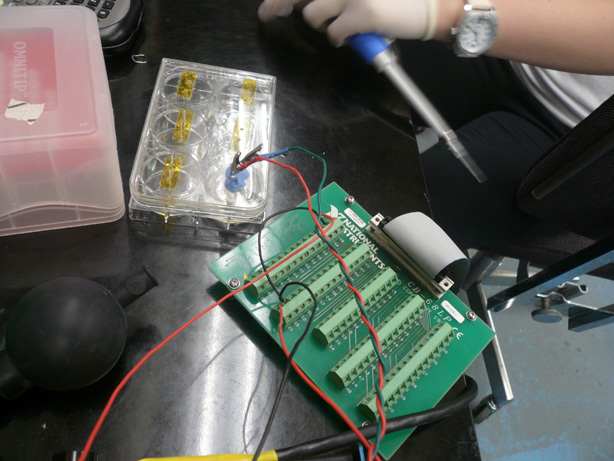
ResistanceTeam Toxipop started off the summer focusing on measuring changes in resistance. Much of our summer work was devoted to the construction of an electrical circuit and measuring apparatus unique to our purpose. Many additions were made to the apparatus each week. Below, you will find information about the different "versions" of the apparatus including final information about our switch to Conductivity. Design V1Design V2We started off the summer with the goal of constructing a measuring apparatus that would offer provide consistent and reliable resistance readings. There were several parameters that needed to be kept constant in order to get good readings. Taken from the equation for resistance measurement, as shown above, we made sure to keep the We made sure to keep the electrodes stationary and at a fixed distant in each different sample of bacteria. Our very first apparatus was made with Legos and poster board. At this point we only had a standard voltmeter that could detect large resistance changes between different salt solutions. The changes we expected to see, however, were on a much smaller scale. Revisions to the First Apparatus
The FINAL Apparatus
You can take readings in units of conductivity (µS/cm) or concentration (mg/L TDS as NaCl). The Conductivity Probe can monitor conductivity at three different sensitivity settings:
Conductivity is directly proportional to concentration over the entire range. The probe has a fast response time, reaching 98% of full value in less than 5 seconds. You can load saved calibrations using any of the Vernier data-collection programs. Alternatively, you can do a quick two-point calibration: the probe is left out of solution for one calibration point (0 µS/cm) and is placed in a known standard (1000 µS/cm solution is provided) for a second calibration point. More precise calibration in two different standards is also possible using our software. The Conductivity Probe uses alternating current at its electrodes; this prevents polarization and electrolysis, so that solutions being tested are not fouled. Corrosion of metal electrodes is not a problem with this epoxy-body graphite electrode. It has built-in temperature compensation, which allows you to do your calibrations in the lab, and then make measurements outdoors without temperature changes affecting conductivity readings. -http://www.vernier.com/probes/con-bta.html |
 "
"