Team:Freiburg Cloning Strategy
From 2008.igem.org
| Line 982: | Line 982: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | + | <br> | |
| - | ==Biobrick fusion protein== | + | |
| + | ==Biobrick fusion protein== | ||
| + | |||
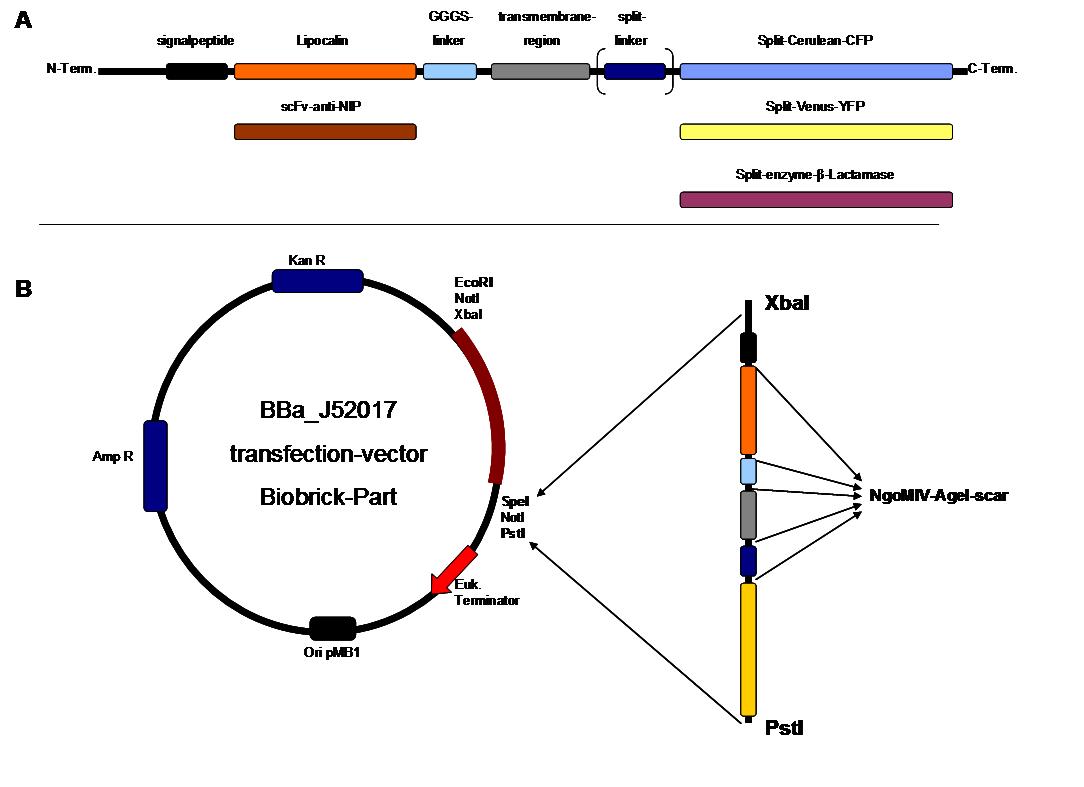
The present BioBrick prefix and suffix rules are not compatible with modular protein design. Thus as in 2007, we propose an extension of the present standard for fusion proteins in which two restriction sites are added in frame adjacent to the coding sequence. The basic parts and as well all composite parts follow this strategy. | The present BioBrick prefix and suffix rules are not compatible with modular protein design. Thus as in 2007, we propose an extension of the present standard for fusion proteins in which two restriction sites are added in frame adjacent to the coding sequence. The basic parts and as well all composite parts follow this strategy. | ||
| - | To get further information see [http://parts.mit.edu/igem07/index.php/Freiburg07/report_fusion_parts FreiGEM07_report_fusion_part] | + | To get further information see [http://parts.mit.edu/igem07/index.php/Freiburg07/report_fusion_parts FreiGEM07_report_fusion_part]<br> |
| + | |||
| + | |||
| + | ==Methods== | ||
| + | |||
| - | |||
| - | |||
The cloning was started with a preparative digestion of the DNA-Plasmids. To clone fusion parts the vector constructs were digested with AgeI and PstI to open the Biobrick suffix. The inserts were digested with NgoMIV and PstI. For cloning into the transfection-vector the enzymes SpeI and PstI were used for vector and XbaI, PstI for insert to keep up the ATG-start codon in the XbaI restriction site of the biobrick suffix. All restriction-enzymes were ordered from New England Biolabs. After digestion the DNA-fragments were separated on a 1% agarose gel. The DNA-band of interest was isolated and purified with the QIAGEN QIAquick Gel Extraction Kit. For the ligation a 3 molar excess of the insert was put together with the vector-fragment and ligated with a Quick ligase (New England Biolabs). After half an our at room temperature the DNA was transformed to chemical competent E.coli strain XL1 cells, plated on 2YT-agar-plates and incubated at 37°C over night. After picking clones and growing in 5ml LB-medium, the plasmid DNA was isolated by QIAGEN QIAprep Spin Miniprep Kit. A test digestion was prepared with about 0,5µg Plasmid DNA and NotI restriction enzyme to isolate the fusion-protein from the vector and to control if the expected bands were obtained. After a positive result the clones were sent to GATC-Biotech for sequencing. | The cloning was started with a preparative digestion of the DNA-Plasmids. To clone fusion parts the vector constructs were digested with AgeI and PstI to open the Biobrick suffix. The inserts were digested with NgoMIV and PstI. For cloning into the transfection-vector the enzymes SpeI and PstI were used for vector and XbaI, PstI for insert to keep up the ATG-start codon in the XbaI restriction site of the biobrick suffix. All restriction-enzymes were ordered from New England Biolabs. After digestion the DNA-fragments were separated on a 1% agarose gel. The DNA-band of interest was isolated and purified with the QIAGEN QIAquick Gel Extraction Kit. For the ligation a 3 molar excess of the insert was put together with the vector-fragment and ligated with a Quick ligase (New England Biolabs). After half an our at room temperature the DNA was transformed to chemical competent E.coli strain XL1 cells, plated on 2YT-agar-plates and incubated at 37°C over night. After picking clones and growing in 5ml LB-medium, the plasmid DNA was isolated by QIAGEN QIAprep Spin Miniprep Kit. A test digestion was prepared with about 0,5µg Plasmid DNA and NotI restriction enzyme to isolate the fusion-protein from the vector and to control if the expected bands were obtained. After a positive result the clones were sent to GATC-Biotech for sequencing. | ||
The GGGS-Linker was produced by Klenow -fill-in-PCR. Two primers were designed align to each other at 60°C and filled to a complete dobble-strand by Klenow Polymerase fragment.<br> | The GGGS-Linker was produced by Klenow -fill-in-PCR. Two primers were designed align to each other at 60°C and filled to a complete dobble-strand by Klenow Polymerase fragment.<br> | ||
| Line 1,031: | Line 1,035: | ||
program: 94°C for 3min, cool down to 37°C, adition of klenow enzyme, 37°C for 1 hour | program: 94°C for 3min, cool down to 37°C, adition of klenow enzyme, 37°C for 1 hour | ||
| - | + | ||
| - | ==Cloned parts== | + | ==Cloned parts== |
| + | |||
The cloning parts scFv-anti-NIP, lipocalin-FluA, Split-Venus-N-YFP, Split-Venus-C-YFP, Cerulean-N-CFP and Cerulean-C-CFP were obtained by genesynthesis and ordered by the GENEART company. The β-Lactamase fragments were adopted from genesynthesis GENEART order in 2007. The erbb1-transmembrane, Split-Fluorophor-Linker and the erbb1-signalpeptide were synthesized by ATG:biosynthetics. The CMV promoter cloning part was produced by PCR with the template J52038 using the CMV_RLuc_Forward-primer and CMV_RLuc_Reverse-primer. To synthesize the GGGS-linker a Klenow-fill-in-reaction was performed by using Klenow-fragment without exonuclease activity and GGGS-forward and reverse primer.<br> | The cloning parts scFv-anti-NIP, lipocalin-FluA, Split-Venus-N-YFP, Split-Venus-C-YFP, Cerulean-N-CFP and Cerulean-C-CFP were obtained by genesynthesis and ordered by the GENEART company. The β-Lactamase fragments were adopted from genesynthesis GENEART order in 2007. The erbb1-transmembrane, Split-Fluorophor-Linker and the erbb1-signalpeptide were synthesized by ATG:biosynthetics. The CMV promoter cloning part was produced by PCR with the template J52038 using the CMV_RLuc_Forward-primer and CMV_RLuc_Reverse-primer. To synthesize the GGGS-linker a Klenow-fill-in-reaction was performed by using Klenow-fragment without exonuclease activity and GGGS-forward and reverse primer.<br> | ||
<br> | <br> | ||
Revision as of 13:08, 29 October 2008
|
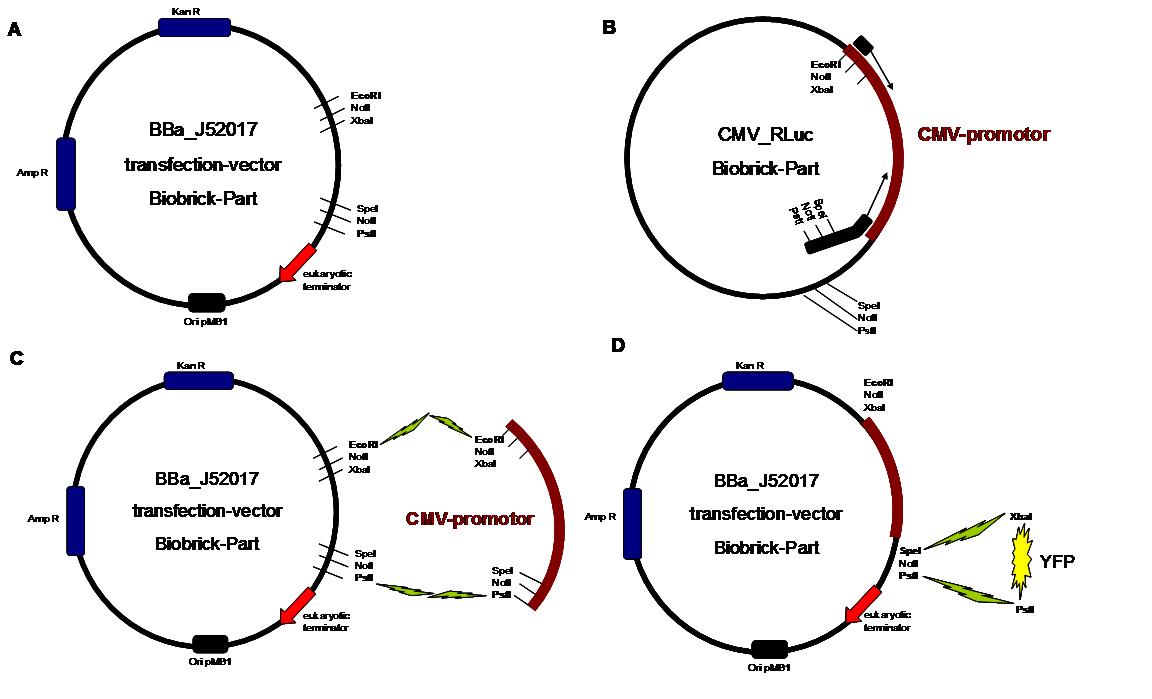
_cloning strategy
Table1_cloning strategy overview about the cloning steps to create the different types of synthetic receptors. To get further information about the composit parts see https://2008.igem.org/Team:Freiburg/Parts
Step 1 Vector digestion: EcoRI + PstI Insert
digestion: EcoRI + PstI BBa-J52017 _CMV-promotor Step
2 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_ SPLIT-Linker C-YFP C-CFP Step
3 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_egfR-Tm _ N-β-Lactamase _ C-β-Lactamase _ SPLIT-Linker_ C-YFP _ N-YFP _ SPLIT-Linker_ C-CFP _ N-CFP _ BB058 (Luciferase) _ BB057 (Luciferase) Step
4 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_SP _scFv-anti-NIP _ Lipocalin Step
5 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_SP_ scFv-anti-NIP and pMA-BBFR-+SP_ Lipocalin _GGGS-linker (produced by Klenow fill in) Step
6 Vector digestion: AgeI+SpeI Insert
digestion: NgoMIV+SpeI pMA-BBFR
_SP_ scFv-anti-NIP _ GGGS-Li and pMA-BBFR
_ SP_ Lipocalin _ GGGS-Li _
egfR-Tm _ N-β-Lactamase _
egfR-Tm _ C-β-Lactamase _
egfR-Tm _ SPLIT-Linker_ C-YFP _
egfR-Tm _ N-YFP _
egfR-Tm _ SPLIT-Linker_ C-CFP _
egfR-Tm _ N-CFP _
egfR-Tm _ BB058 (Luciferase) _
egfR-Tm _ BB057 (Luciferase) Step
7 Vector digestion:
SpeI + PstI Insert
digestion: XbaI + PstI BBa-J52017_CMV _SP_ scFv-anti-NIP_GGGS-Li_egfR-Tm_N-β-Lactamase _ SP_ scFv-anti-NI _GGGS-Li_ egfR-Tm_C-β-Lactamase _ SP_ scFv-anti-NIP_GGGS-Li_
egfR-Tm_SPLIT-Linker_C-YFP _ SP_ scFv-anti-NIP_GGGS-Li_ egfR-Tm_N-YFP _ SP_ scFv-anti-NIP_GGGS-Li_
egfR-Tm_SPLIT-Linker_C-CFP _ SP_ scFv-anti-NIP_GGGS-Li_ egfR-Tm_N-CFP _ SP_ scFv-anti-NIP_GGGS-Li _ egfR-Tm_BB058 (Luciferase) _ SP_ scFv-anti-NIP_GGGS-Li _ egfR-Tm_BB057 (Luciferase) _ SP_ Lipocalin _GGGS-Li_ egfR-Tm_N-β-Lactamase _ SP_ Lipocalin _GGGS-Li_ egfR-Tm_C-β-Lactamase _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_SPLIT-Linker_ C-YFP _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_N-YFP _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_SPLIT-Linker_ C-CFP _ SP_ Lipocalin _GGGS-Li_
egfR-Tm_N-CFP _ SP_ Lipocalin _GGGS-Li__ egfR-Tm _ BB058 (Luciferase) _ SP_ Lipocalin _GGGS-Li__ egfR-Tm _ BB057 (Luciferase) The present BioBrick prefix and suffix rules are not compatible with modular protein design. Thus as in 2007, we propose an extension of the present standard for fusion proteins in which two restriction sites are added in frame adjacent to the coding sequence. The basic parts and as well all composite parts follow this strategy.
To get further information see [http://parts.mit.edu/igem07/index.php/Freiburg07/report_fusion_parts FreiGEM07_report_fusion_part] The cloning was started with a preparative digestion of the DNA-Plasmids. To clone fusion parts the vector constructs were digested with AgeI and PstI to open the Biobrick suffix. The inserts were digested with NgoMIV and PstI. For cloning into the transfection-vector the enzymes SpeI and PstI were used for vector and XbaI, PstI for insert to keep up the ATG-start codon in the XbaI restriction site of the biobrick suffix. All restriction-enzymes were ordered from New England Biolabs. After digestion the DNA-fragments were separated on a 1% agarose gel. The DNA-band of interest was isolated and purified with the QIAGEN QIAquick Gel Extraction Kit. For the ligation a 3 molar excess of the insert was put together with the vector-fragment and ligated with a Quick ligase (New England Biolabs). After half an our at room temperature the DNA was transformed to chemical competent E.coli strain XL1 cells, plated on 2YT-agar-plates and incubated at 37°C over night. After picking clones and growing in 5ml LB-medium, the plasmid DNA was isolated by QIAGEN QIAprep Spin Miniprep Kit. A test digestion was prepared with about 0,5µg Plasmid DNA and NotI restriction enzyme to isolate the fusion-protein from the vector and to control if the expected bands were obtained. After a positive result the clones were sent to GATC-Biotech for sequencing.
The GGGS-Linker was produced by Klenow -fill-in-PCR. Two primers were designed align to each other at 60°C and filled to a complete dobble-strand by Klenow Polymerase fragment. The cloning parts scFv-anti-NIP, lipocalin-FluA, Split-Venus-N-YFP, Split-Venus-C-YFP, Cerulean-N-CFP and Cerulean-C-CFP were obtained by genesynthesis and ordered by the GENEART company. The β-Lactamase fragments were adopted from genesynthesis GENEART order in 2007. The erbb1-transmembrane, Split-Fluorophor-Linker and the erbb1-signalpeptide were synthesized by ATG:biosynthetics. The CMV promoter cloning part was produced by PCR with the template J52038 using the CMV_RLuc_Forward-primer and CMV_RLuc_Reverse-primer. To synthesize the GGGS-linker a Klenow-fill-in-reaction was performed by using Klenow-fragment without exonuclease activity and GGGS-forward and reverse primer. |
 "
"