Team:Freiburg Calcium Imaging
From 2008.igem.org
(Difference between revisions)
WeberSimone (Talk | contribs) |
|||
| (34 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
Content= | Content= | ||
<div style="font-size:18pt;"> | <div style="font-size:18pt;"> | ||
| - | <font face="Arial Rounded MT Bold" style="color:#010369">_Cell Stability, | + | <font face="Arial Rounded MT Bold" style="color:#010369">_Cell Stability, Ca<sup>2+</sup> Signaling and DNA-Origami Binding to Cells</font></div> |
| - | |||
| + | <h1>Introduction</h1> | ||
| + | To test receptor activation in a natural context, it was also tried to | ||
| + | activate T-cells (B12.7.5) with the NIP-linked DNA-origami. Those | ||
| + | T-cells have a NIP Fab-fragment genetically fused to their receptor. | ||
| + | During these tests many problems were faced which could emerge as | ||
| + | obstacles in the main project, the artificial receptor, which is | ||
| + | expressed by 293T-cells.<br> | ||
| + | One problem was to find a medium in which the T-cells survive, the | ||
| + | DNA-Origami structures are stable and which is also suitable for the | ||
| + | fluorescent measurement on the microsope). Normally, the cells were kept | ||
| + | in RPMI (10% FCS), but the phenol red itself is an electron acceptor | ||
| + | and would disturb the measurement. Another complication was, that the | ||
| + | Origami need a high Mg<sup>2+</sup> concentration (12.5 mM), which stabilizes the | ||
| + | DNA backbone, but low concentration of other bivalent cations, which | ||
| + | could disrupt the Origami. None of the common cell culture medium does | ||
| + | achieve these conditions. In order to solve the cell culture problem | ||
| + | the stability of the Origami in different media were tested (see | ||
| + | DNA-Origami). On the other hand we also had to test if our cells | ||
| + | survive 12.5 mM Mg<sup>2+</sup>, which we tested with an MTT-Assay. <br> | ||
| + | As explained before, we also wanted to use the Origami to activate | ||
| + | T-cell receptors (TCR) by clustering. For this experiment we measured | ||
| + | the calcium influx with a FACS, as described earlier (Susana Minguet, | ||
| + | Immunity Vol. 26, Page 43-54). But in this publication much higher | ||
| + | concentration of the stimulus were used than we were able to produce. | ||
| + | Therefore we were looking for another method to measure the calcium | ||
| + | influx. One very commonly used method is to stain the cells with | ||
| + | Fura-2AM and measure the changes in calcium concentration with a | ||
| + | confocal laser scanning microscope. Usually this measurement is only | ||
| + | suitable for adherent cells, because by giving the stimulus to the | ||
| + | cells the cells would move. To avoid this problem we used Poly-L-Lysin | ||
| + | coated µ-Slides(ibidi). <br> | ||
| + | To check, that the DNA-Origami really bind specifically to the | ||
| + | cells(T-cells and B-cells), Alexa 488 linked Origamis with and without | ||
| + | NIP were given to the cells and the fluorescence was visualized with a | ||
| + | LSM.<br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <h1>Material and Methods</h1> | ||
| + | <br> | ||
| + | <h2>Cell stability in the presence of Mg<sup>2+</sup> measured by MTT-Assay</h2> | ||
| + | To test the Mg<sup>2+</sup> tolerance of the T-cells (cell line | ||
| + | B.12.7.5), 100 µl cellsuspension was mixed with 800 µl RPMI | ||
| + | medium and 100 µl MgCl2 or MgAc, respectively | ||
| + | containing various concentrations of Mg<sup>2+</sup> in a 24-well plate. 3 days | ||
| + | later cells of each well were spun down, the supernatant was | ||
| + | discarded and the cells were resuspended in 200 µl new RPMI medium. 50 | ||
| + | µl 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid (MTT) was | ||
| + | added to each sample. After 4 h of incubation at 37°C the cells were | ||
| + | spun down again and after discarding the medium the pellet was resolved | ||
| + | in 400 µl DMSO and 50 µl Soerensens’ reagent. The reduced blue MTT was | ||
| + | detected in a photometer at 570nm.<br> | ||
| + | <br> | ||
| + | B-cells (cell line j558lδmmb1nfleck) from 10 ml dishes were | ||
| + | spun down and resuspended in 9ml Krebs-Ringer-Hepes (12.5mM MgAc). | ||
| + | After incubation for 45 min the cells were spun down again and resolved | ||
| + | in 1 ml PBS. 5 µl of the suspension was mixed with 45 µl Trypan blue and | ||
| + | the cells were counted in a “Neubauer cell chamber”.<br> | ||
| + | <br> | ||
| + | |||
| + | 293T cells were scraped off an 10 ml dish, spun down and resolved in 10 ml new DMEM medium. 500 µl of this suspension was given in each plate of a 6-well plate containing 4500 µl DMEM medium with different concentrations of Mg<sup>2+</sup>. 3 days later the media of 3 wells was sucked off and the cells were washed in PBS, then TA-buffer (Tris-Acetat buffer) was given to these wells. After 1h the TA-buffer was removed, the cells of all dishes were washed in PBS and 2ml new DMEM medium plus 500 µl MTT was added. After incubation for 3.5h at 37°C the cells were scraped off the wells and spun down at 13000 rpm for 5min. Then the pellet was resolved in 4 ml DMSO and 500 µl Soerensens’ reagent. Detection took place at 570 nm.<br> | ||
| + | <br> | ||
| + | <h2>Media</h2> | ||
| + | <br> | ||
| + | Medium for B- and T-cell :<br> | ||
| + | <ul> | ||
| + | <li>RPMI</li> | ||
| + | <li>10% FCS </li> | ||
| + | <li>HEPES (10mM) </li> | ||
| + | <li>β-mercaptoethanol (50µM) </li> | ||
| + | <li>L-Glutamine (2 mM)</li> | ||
| + | <li>1% Pen-Strep</li> | ||
| + | </ul> | ||
| + | <br> | ||
| + | Medium for 293T:<br> | ||
| + | <ul> | ||
| + | <li>DMEM</li> | ||
| + | <li>10% FCS </li> | ||
| + | <li>5% PenStrep </li> | ||
| + | <li>L-Glutamine (1.5 mM) <br> | ||
| + | </li> | ||
| + | </ul> | ||
| + | <br> | ||
| + | Krebs-Ringer-Hepes (12.5 mM Mg<sup>2+</sup>):<br> | ||
| + | <ul> | ||
| + | <li>NaCl (155 mM)</li> | ||
| + | <li>KCl (4.5 mM)</li> | ||
| + | <li>CaCl2 (2 mM)</li> | ||
| + | <li>MgCl2 (1 mM)</li> | ||
| + | <li>MgAcetat (11.5 mM)</li> | ||
| + | <li>D-glucose (10 mM)</li> | ||
| + | <li>Hepes (5 mM)</li> | ||
| + | </ul> | ||
| + | -> pH 7.4 with NaOH<br> | ||
| + | <br> | ||
| + | Soerensens’ reagent:<br> | ||
| + | <ul> | ||
| + | <li>10 ml glycine (0.1 M)</li> | ||
| + | <li>10 ml NaCl (0.1 M)</li> | ||
| + | <li>80 ml Aqua dest.</li> | ||
| + | </ul> | ||
| + | -> pH 10.5 with NaOH<br> | ||
| + | <br> | ||
| + | <h2>Binding measurement</h2> | ||
| + | To test the binding between origamis and T-cells/B-cells 15 µl cell | ||
| + | suspension in Ringer (12.5 mM Mg<sup>2+</sup>) or TA-buffer (12.5 mM Mg<sup>2+</sup>) was mixed | ||
| + | with 15 µl of origamis on a µ-Slide (ibidi, µ-Slides 18 well-flat, Cat. | ||
| + | No: 81824). Those slides are coated with Poly-L-Lysine, which fixes the | ||
| + | cells on the bottom of the slide. So the suspensions cells could be | ||
| + | measured on a microscope. <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <h2>Calcium<sup>2+</sup> measurement</h2> | ||
| + | <br> | ||
| + | <h3>Ca<sup>2+</sup> measurement with microscope</h3> | ||
| + | By binding of ligands to a receptor at the cell surface the cell reacts | ||
| + | amongst others with a efflux of calcium ions from the ER into the | ||
| + | cytoplasm. To measure the intensity of activation one way is to | ||
| + | quantify the concentration or rather the increase of calcium ions in the | ||
| + | cytoplasm. Fura-2 is a fluorescent dye which change the quality | ||
| + | dependent on the Ca<sup>2+</sup> concentration. Fura-2AM (Fura-2-acetoxymethyl | ||
| + | ester) is a membrane-permeable derivative of Fura-2 but after crossing | ||
| + | the membrane the acetoxymethyl groups are removed by cellular esterases | ||
| + | so it remains as Fura-2 in the cytoplasm. Fura-2 is excited at 340 nm | ||
| + | and 380 nm of light, and the ratio of the emissions at those | ||
| + | wavelengths is directly correlated to the amount of intracellular | ||
| + | calcium. Without Ca<sup>2+</sup> the maximum emission results from excitation at | ||
| + | 365nm. With Ca<sup>2+</sup> the maximum emission change to excitation at 340 nm and | ||
| + | the emission decrease by extinction at 380 nm.<br> | ||
| + | So to measure properly it is necessary to alternate quickly between the | ||
| + | two excitation wavelengths. Excitation was measured with a high-end | ||
| + | inverted fluorescenc microscope (Zeiss Axiovert 100). <br> | ||
| + | <br> | ||
| + | <br> | ||
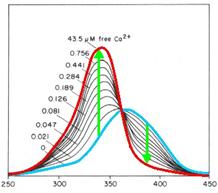
| + | [[Image:TeamFreiburg2008_FURA_PRINZIP.jpg]]<br> | ||
| + | <small>Fig. 1: Fura-2 Emission with (blue) and without free | ||
| + | calciumions (red)</small><br><br> | ||
| + | '''Fura-2 Loading'''<br> | ||
| + | <u>Procedure:</u><br><br> | ||
| + | 1. 1 ml T-cell cellsuspension (in Ringer-Solution)<br> | ||
| + | 2. + 0.5 µl Pluronic F 127<br> | ||
| + | 3. + 0.5 µl Fura-Solution (2mM) and vortex<br> | ||
| + | 4. incubation at 37°C and 8% CO2 for 20 min <br> | ||
| + | 5. centrifuge at 1000 rpm (206 rcf) and 4°C for 5 min<br> | ||
| + | 6. discard the supernatant<br> | ||
| + | 7. + 100 µl Ringer-Solution for resuspension<br> | ||
| + | <br> | ||
| + | <h3>Ca<sup>2+</sup> measurement with FACS</h3> | ||
| + | Cells resuspended in medium with 1% serum were incubated with 5 μg/ml | ||
| + | of Indo-1, which is the Ca<sup>2+</sup> complexing dye, and 0.5 μg/ml of | ||
| + | pluronic F-127, which fasilitates dye uptake (both Molecular Probes) 45 | ||
| + | min at 37°C. After incubation, cells were distributed into to 1.5 ml | ||
| + | eppendorf tubes and the washed with the medium we wanted to measure | ||
| + | them. After washing, cells were resuspended in the according medium and | ||
| + | kept on ice. Ca<sup>2+</sup> response was induced by addition of the indicated | ||
| + | stimulus 1 min after starting to record the ratio of Ca<sup>2+</sup>-bound Indo-1 | ||
| + | versus unbound Indo-1 with a LSRII fluorescence spectrometer (Becton | ||
| + | Dickinson). Cells were measured for approximately 2min before putting | ||
| + | the stimuli on it. Data were analyzed with the FloJo 6.1 software.<br> | ||
| + | <br> | ||
| + | <h1>Results and discussion</h1> | ||
| + | <h2>Cell stability in the presence of Mg<sup>2+</sup> measured by MTT-Assay</h2> | ||
| + | '''T-cells:'''<br> | ||
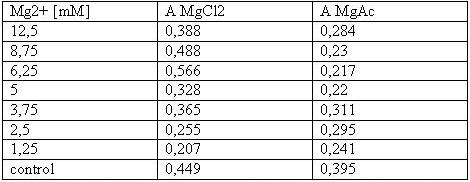
| + | [[Image:Freiburg08tabellet-cellstability.JPG|Freiburg08tabellet-cellstability.JPG]]<br> | ||
| + | <small>Table 1: Absorbance of reduced MTT of T-cells with various Mg<sup>2+</sup> concentration</small><br> | ||
| + | <br> | ||
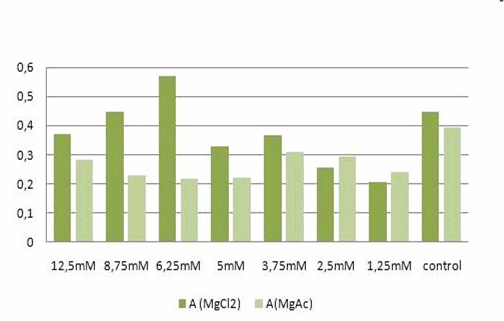
| + | [[Image:TeamFreiburg2008-t-cellstability1.png]]<br> | ||
| + | <small>Fig. 2: graphic illustration of the results from Table 1.</small><br> | ||
| + | <br> | ||
| + | '''B-cells:'''<br> | ||
| + | Counting stained and unstained B-cells brought following result: <br> | ||
| + | Dead cells : 1<br> | ||
| + | Living cells: 44<br> | ||
| + | Total cell number: 45<br> | ||
| + | <br> | ||
| + | '''293T-cells:'''<br> | ||
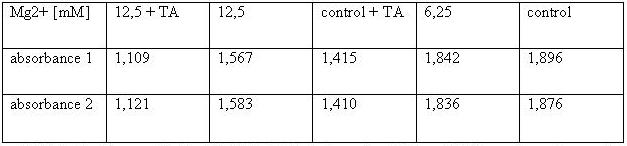
| + | [[Image:TeamFreiburg2008_TABELLE-t-cellstability3.jpg ]]<br> | ||
| + | <small>Table 2: Absorbance of reduced MTT of 293T-cells with various Mg<sup>2+</sup> concentration and TA treatment</small><br> | ||
| + | <br> | ||
| + | The MTT assays and the trypan blue staining proofed the tolerance of | ||
| + | the used cells towards a concentration up to 12.5 mM Mg<sup>2+</sup>. This is the | ||
| + | exact concentration in which the origami are produced and stored. The | ||
| + | lower absorbance in the tests with TA could possibly come from the | ||
| + | removal of the TA-buffer because it seemed that the TA buffer disturbs | ||
| + | the adhesion of the 293T cells to the ground of the well so that some | ||
| + | cells might be sucked off with the TA.<br> | ||
| + | <br> | ||
| + | <h2>Calcium<sup>2+</sup> measurement</h2> | ||
| + | <h3>Ca<sup>2+</sup> measurement with FACS</h3> | ||
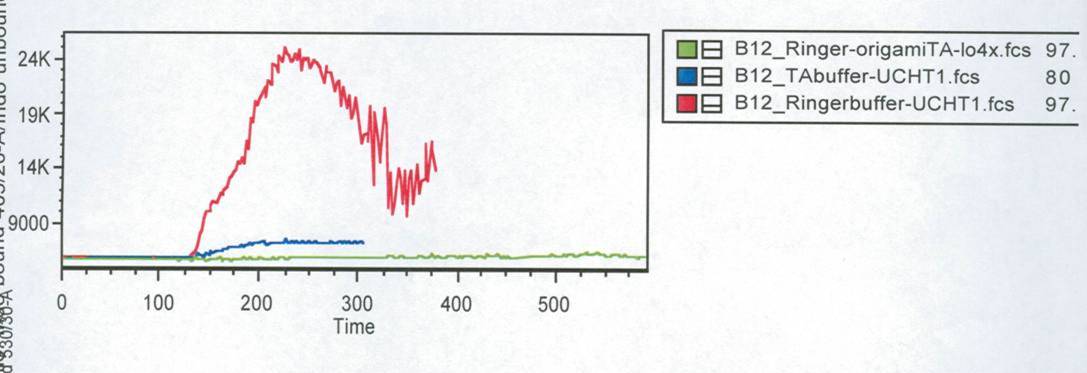
| + | In this measurement we tried to activate T-Cells by clustering. | ||
| + | Therefore we tested two different buffers, Krebs-Ringer-Hepes with | ||
| + | 12.5 mM Mg<sup>2+</sup> buffer and TA with 12.5 mM Mg<sup>2+</sup>. As positive control we used | ||
| + | UCHT1 (=anti-CD3), which can stimulate T-cells (Susana Minguet, Vol. | ||
| + | 26, Page 43-54).<br> | ||
| + | <br> | ||
| + | <br> | ||
| + | [[Image:FACS-Auswertung_fuer_final_report.jpg|600 px]]<br> | ||
| + | <small>Fig. 3: Results of the FACS measurement. Cells were stained with | ||
| + | Indo-1. Different stimuli were used. Stimuli were given after 1min. | ||
| + | Time is given in seconds.<br> | ||
| + | Green line: cells buffered in | ||
| + | Krebs-Ringer-Hepes buffer. The both other lines show the positive | ||
| + | controls of cells buffered in TA (blue) and Krebs-Ringer-Hepes (red).</small><br> | ||
| + | <br> | ||
| + | Figure 3 shows the change in intra cellular calcium concentration after | ||
| + | adding the DNA-Origami (green) compared to the positive controls (blue | ||
| + | and red). The cells in Krebs-Ringer-Hepes buffer, which were stimulated | ||
| + | with UCHT 1 show a very high activation, while the cells buffered in TA | ||
| + | and the Origami treaded cell show only a slowly signal. When we put the | ||
| + | cells buffered in TA in the LSRII fluorescence spectrometer and | ||
| + | rechecked all the settings we saw, that most of the cells were already | ||
| + | dead. This could be the reason for the low signal of the TA buffered | ||
| + | cells (blue line). The cells treaded with Origami didn´t show any | ||
| + | calcium change at all. After the FACS measurement we tested some of | ||
| + | those Origami on the AFM. None of the Origami which we used were | ||
| + | stable, so probably that is the reason why we couldn´t see any signal | ||
| + | by adding NIP-Origami. To confirm this conclusion this measurement would | ||
| + | have to be carried out again.<br> | ||
| + | <br> | ||
| + | <h3>Ca<sup>2+</sup> measurement with microscope</h3> | ||
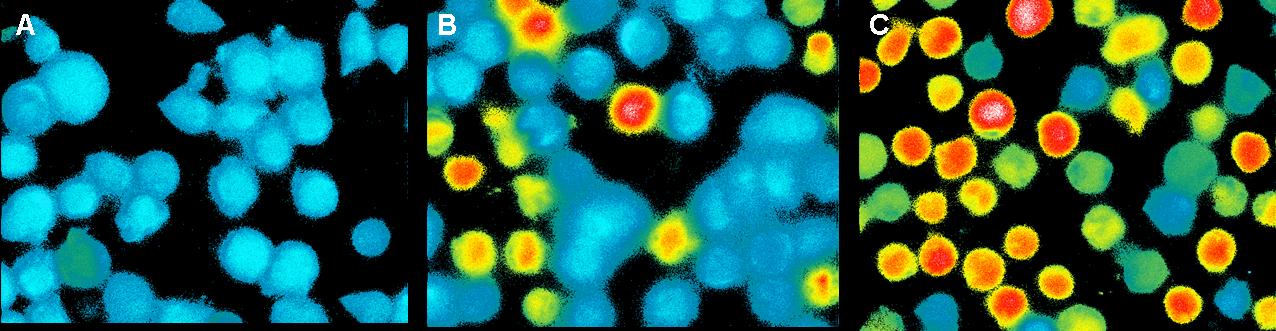
| + | This measurement was also used to activate the T-cell receptors (TCR) | ||
| + | by clustering. The TCR's were modified with a anti-NIP antibodies and | ||
| + | the NIP-molecules were coupled to DNA origamis. As a negative control | ||
| + | we used a DNA-Origami without NIP. The positive control was Pervanadat.<br> | ||
| + | By adding the origamis to the cells we could see a small signal for | ||
| + | both with and without NIP. The amount of fluorescent cells increase | ||
| + | slowly but there was no significant difference between the negative | ||
| + | control and the sample (7 NIP's per origami).<br> | ||
| + | By contrast the Pervanadat produced a strong signal that differs from | ||
| + | the origami reactions.<br> | ||
| + | <br> | ||
| + | [[Image:CalciumImaging_teamfreiburg2008.jpg|750px]]<br> | ||
| + | <small>Fig. 4: T-cells stimulated with Pervanadat: A) 1 sec before addition B) 100 sec after addition C) 200 sec after addition</small><br> | ||
| + | <br><br> | ||
| + | In contrast to the positive control (Pervanadat) which was working quit | ||
| + | well, our sample (DNA-origami with NIP) and the negative control | ||
| + | (DNA-origami without NIP) did not show a significant Ca<sup>2+</sup> efflux. There | ||
| + | are two reasons which could be responsible that the cell answer to | ||
| + | origamis with and without NIP’s almost looks the same.<br> | ||
| + | 1. Spatial avoidance: Because of the rough surface of T-cells big | ||
| + | molecules could have problems to trigger two or more TCR’s. Also huge | ||
| + | extracellular proteins could avoid that the nanoplates reach the small | ||
| + | TCR’s.<br> | ||
| + | 2. Non-specific binding:<br> | ||
| + | Also the negative control produced a weak signal which could result | ||
| + | from non-specific binding to the cell surface, because the cells were | ||
| + | not blocked.<br> | ||
| + | <br> | ||
| + | In both cases the slow Ca<sup>2+</sup> efflux could result from the mechanical | ||
| + | touch between the cells by adding the liquid with the probes.<br> | ||
| + | <h2>Binding measurement</h2><br> | ||
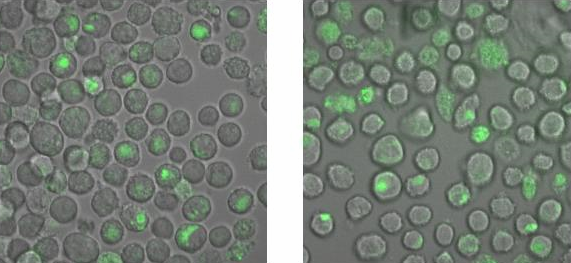
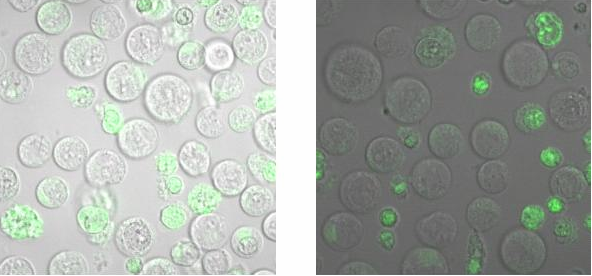
| + | To check, that the DNA-Origami really bind specifically to the T-cells and B-cells, both genetically fused to a NIP Fab-fragment, Alexa 488 linked origamis with and without NIP were given to the cells and the fluorescence was visualized with a LSM. The results are shown in Figure 4 and 5.<br> | ||
| + | <br> | ||
| + | [[Image:TeamFreiburg2008_Bindungsmessung_1.jpg|600 px]]<br> | ||
| + | <small>Fig. 5: B-cells with NIP linked Origami (left) and without NIP (right) in 50% TA (Tris-Acetat) and 50% Krebs-Ringer-Hepes buffer, </small><br> | ||
| + | <small>both buffers contain 12.5 mM Mg<sup>2+</sup> </small> | ||
| + | <br> | ||
| + | <br> | ||
| + | [[Image:Freiburg2008_Bindungsmessung2.PNG| 600px]]<br> | ||
| + | <small>Fig. 6: B-cells with NIP linked Origami (left) and without NIP (right) in TA (Tris-Acetat)</small><br> | ||
| + | <br> | ||
| + | Figure 5 and 6 show that the Origami with NIP as well as the Origami without NIP bound to both cell types. During the binding measurements it seemed that the origamis were | ||
| + | absorbed by the cells or that they bind unspecifically. Later tests at | ||
| + | the AFM, with the same Origami showed no functional origami which could be an explanation to | ||
| + | the behaviour of the cells. The expanded form of the B-cells in | ||
| + | TA-buffer showed that sole TA-buffer is osmotically disadvantageous for | ||
| + | the cells.<br> | ||
}} | }} | ||
Latest revision as of 00:58, 30 October 2008
 "
"