Team:Paris/Construction
From 2008.igem.org
(→Synchronization module) |
|||
| (12 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Paris/Menu}} | {{Paris/Menu}} | ||
| - | + | {{Paris/Header|Model constructions: from the modelling to the characterization}} | |
| + | |||
| + | '''Have a look at our [[Team:Paris/Notebook|notebook]].''' | ||
| + | |||
Our project BacterioClock is based on an oscillating FIFO synchronized at the population level. To obtain and have preliminary results of this system we divided it in three "little" modules as the modeling team exposed them previously. | Our project BacterioClock is based on an oscillating FIFO synchronized at the population level. To obtain and have preliminary results of this system we divided it in three "little" modules as the modeling team exposed them previously. | ||
| - | + | =The First is to demonstrate the [[Team:Paris/Analysis#FIFO_behaviour|FIFO]] which constitutes our core system= | |
| - | A simple manner is to implement two class II | + | A simple manner is to implement two class II promoter followed by fluorescent reporter genes like GFP in a bacteria strain that is able to produce the flagella. |
By this way, we are able to see in single cell the order of the activation of pFliL then pFlgA then pFlhB and then the inactivation in the same order as a FIFO will do. | By this way, we are able to see in single cell the order of the activation of pFliL then pFlgA then pFlhB and then the inactivation in the same order as a FIFO will do. | ||
| Line 12: | Line 15: | ||
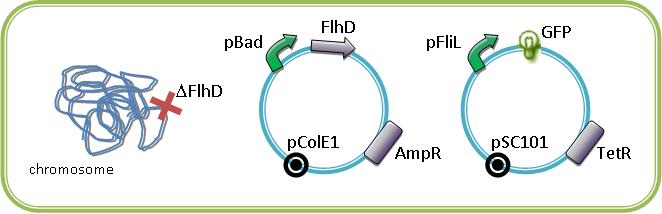
A better way to observe the FIFO is to study the system with an inducible regulator of class II gene in a bacteria strain deleted for this gene. We are extremely grateful for Alon U. that send us the inducible gene ''FlhDC'', and ''FliA'' in pBad18 plasmid. | A better way to observe the FIFO is to study the system with an inducible regulator of class II gene in a bacteria strain deleted for this gene. We are extremely grateful for Alon U. that send us the inducible gene ''FlhDC'', and ''FliA'' in pBad18 plasmid. | ||
| - | These plasmids with our own araC/pBad-EnvZ* will allowed us to study the system in mutated strains that we have in our lab (ΔFlhD, ΔFliA, ΔFlgM, ΔEnvZ). By cotransforming in mutated strains the | + | These plasmids with our own araC/pBad-EnvZ* will allowed us to study the system in mutated strains that we have in our lab (ΔFlhD, ΔFliA, ΔFlgM, ΔEnvZ). By cotransforming in mutated strains the inducible regulators of the class II promoters with one of this promoter associated to a fluorescent protein, we could characterize the influence of the master regulators of the flagella on their promoter. The fluorescence is normalized to the OD<sub>600</sub>. |
<br>For example below are some constructions to see and induce the FIFO: | <br>For example below are some constructions to see and induce the FIFO: | ||
<br><br><br> | <br><br><br> | ||
[[Image:Felipe01.jpg|500px|center|thumb|pFliL alone]]<br><br><br> | [[Image:Felipe01.jpg|500px|center|thumb|pFliL alone]]<br><br><br> | ||
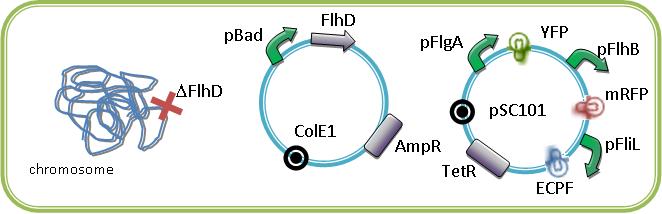
[[Image:Felipe02.jpg|500px|center|thumb|3 promoters of the FIFO in one Cell]]<br><br><br> | [[Image:Felipe02.jpg|500px|center|thumb|3 promoters of the FIFO in one Cell]]<br><br><br> | ||
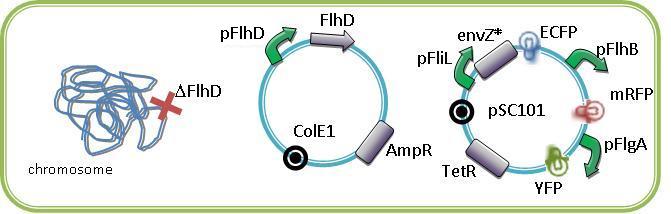
| - | [[Image:Felipe03.jpg|500px|center|thumb|FIFO and oscillating | + | [[Image:Felipe03.jpg|500px|center|thumb|FIFO and oscillating constructions in a single cell due to the flhDC repression by EnvZ*. ]]<br><br><br> |
To perform such a cotransformation we take care about the ORI of each low copy plasmid which are often incompatible and we made all our final constructions in the pSB4T5 plasmid that care the only one resistance that was not already used and the pSC101 ORI which is compatible with the ColE1. | To perform such a cotransformation we take care about the ORI of each low copy plasmid which are often incompatible and we made all our final constructions in the pSB4T5 plasmid that care the only one resistance that was not already used and the pSC101 ORI which is compatible with the ColE1. | ||
| - | We could see the FIFO in a more convenient manner with the assembly of two class II | + | We could see the FIFO in a more convenient manner with the assembly of two class II promoters associated to different fluorophores. Then we could observed two kinds of fluorescents in One Cell along time. |
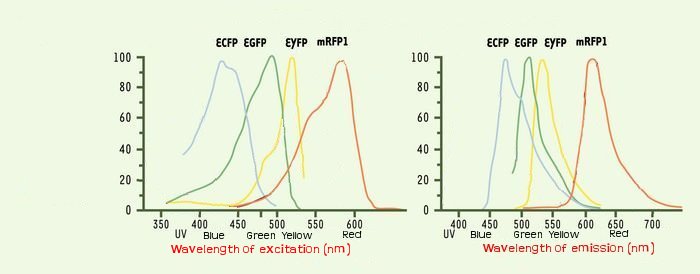
| - | One problem that could occur is the interaction between the | + | One problem that could occur is the interaction between the different fluorescents proteins due to the cross-over of their emission and excitation spectra and moreover in our final system that count three fluorophores. |
[[Image:Spectre.jpg |600px |thumb| emission and excitation spectra of ECFP, YFP and mRFP]] | [[Image:Spectre.jpg |600px |thumb| emission and excitation spectra of ECFP, YFP and mRFP]] | ||
| Line 37: | Line 40: | ||
<br> | <br> | ||
| - | + | =[[Team:Paris/Analysis#Oscillations|Oscillating]] module= | |
| - | Few approaches have been thought in order to create the oscillating module. The pTet/TetR system that allows us to precisely control the inhibition of TetR on the Tet | + | Few approaches have been thought in order to create the oscillating module. The pTet/TetR system that allows us to precisely control the inhibition of TetR on the Tet promoter. Another system use the natural inhibition of OmpR and of EnvZ via OmpR. We finally perform this system that we have developed since we know thanks to the modeling team that the oscillations are the only way to see the full development of the FIFO. |
| + | |||
| + | =Synchronization module= | ||
| + | The modeling simulation showed us that to have some oscillations that are difficult to obtain in a simple feedforward loop without the delay introduce by the quorum sensing synchronization, and for us it is more interesting to have the FIFO behavior at the population level. We did not achieved this module but we started the construction of it and have already some intermediate parts that you will find below. | ||
| - | = | + | =The constructions we were able to obtain were characterised by fluorescence microscopy (see examples below):= |
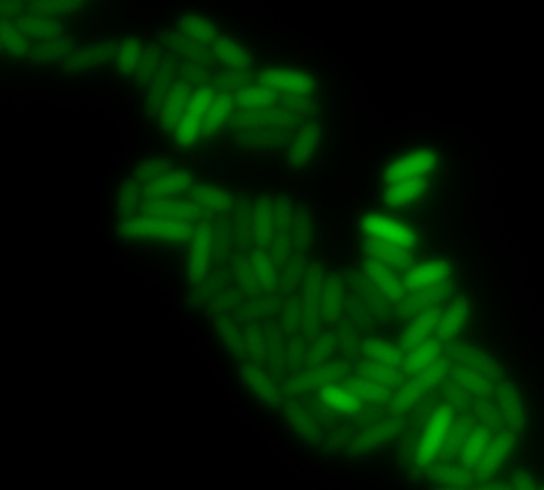
| - | + | [[Image:ParisGfp.jpg|thumb|300px|center|Green fluorescence]] | |
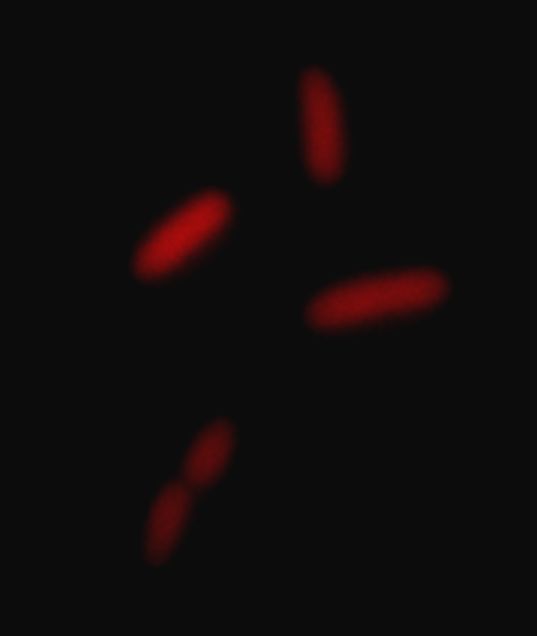
| - | The constructions we were able to obtain | + | [[Image:ParisRfp.jpg|thumb|300px|center|Red Fluorescence]] |
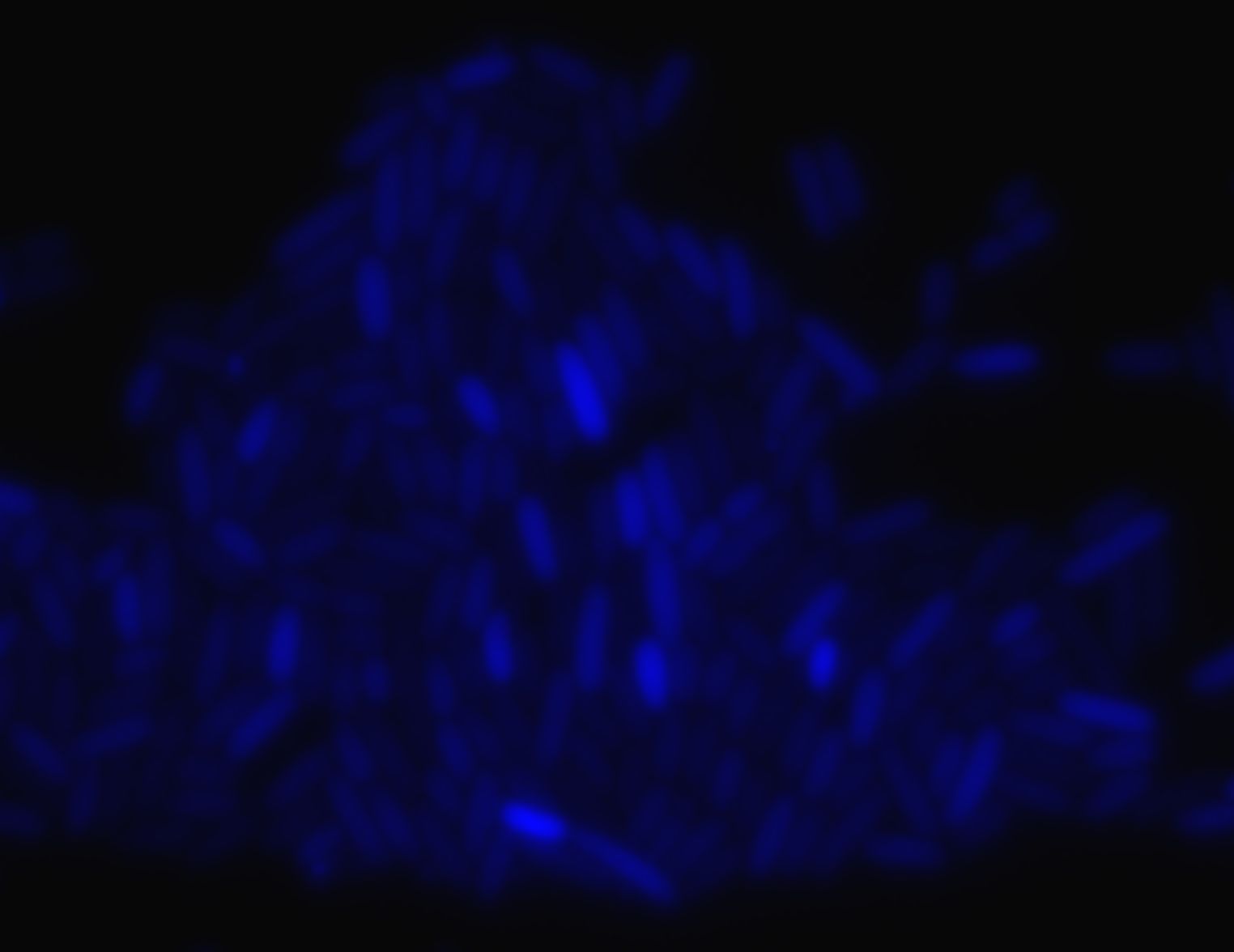
| + | [[Image:ParisCfp.jpg|thumb|300px|center|Cyan Fluorescence]] | ||
| - | + | = Table of the constructions we realized= | |
{| | {| | ||
|- style="background: #649cd7;" | |- style="background: #649cd7;" | ||
Latest revision as of 07:31, 30 October 2008
|
Model constructions: from the modelling to the characterization
Have a look at our notebook.
The First is to demonstrate the FIFO which constitutes our core systemA simple manner is to implement two class II promoter followed by fluorescent reporter genes like GFP in a bacteria strain that is able to produce the flagella. By this way, we are able to see in single cell the order of the activation of pFliL then pFlgA then pFlhB and then the inactivation in the same order as a FIFO will do. These constructions are equivalent to the experiments realized by Uri Alon in the article "Using quantitative Blueprint to reprogram the Dynamics of the Flagella Gene Network" Kalir S, Alon U. Cell 2004. A better way to observe the FIFO is to study the system with an inducible regulator of class II gene in a bacteria strain deleted for this gene. We are extremely grateful for Alon U. that send us the inducible gene FlhDC, and FliA in pBad18 plasmid.
These plasmids with our own araC/pBad-EnvZ* will allowed us to study the system in mutated strains that we have in our lab (ΔFlhD, ΔFliA, ΔFlgM, ΔEnvZ). By cotransforming in mutated strains the inducible regulators of the class II promoters with one of this promoter associated to a fluorescent protein, we could characterize the influence of the master regulators of the flagella on their promoter. The fluorescence is normalized to the OD600.
We could see the FIFO in a more convenient manner with the assembly of two class II promoters associated to different fluorophores. Then we could observed two kinds of fluorescents in One Cell along time. One problem that could occur is the interaction between the different fluorescents proteins due to the cross-over of their emission and excitation spectra and moreover in our final system that count three fluorophores.
We thank a lot Arnau Montagu, advisor of the iGEM Valencia Team. He advised us, after his team had troubleshooting with fluorescence, to take care about spurious FRET. This phenomenon occurs even with non covered fluorescent spectra when the concentration of two fluorophores is sufficient so they are enough closer to induce some FRET. But it could happen at lower concentration than 40 μM.
Oscillating moduleFew approaches have been thought in order to create the oscillating module. The pTet/TetR system that allows us to precisely control the inhibition of TetR on the Tet promoter. Another system use the natural inhibition of OmpR and of EnvZ via OmpR. We finally perform this system that we have developed since we know thanks to the modeling team that the oscillations are the only way to see the full development of the FIFO. Synchronization moduleThe modeling simulation showed us that to have some oscillations that are difficult to obtain in a simple feedforward loop without the delay introduce by the quorum sensing synchronization, and for us it is more interesting to have the FIFO behavior at the population level. We did not achieved this module but we started the construction of it and have already some intermediate parts that you will find below. The constructions we were able to obtain were characterised by fluorescence microscopy (see examples below):Table of the constructions we realized
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"