|
← Yesterday ↓ Calendar ↑Tomorrow →
Sequencing Minipreps we did yesterday
- O18 has been diluted to have a concentration of 5µM
- In each tube : 1µL of O18 diluted
- Pure water qsp 15µL
| Name
| Ligation
| Concentration MP (µg/mL)
| Vol MP (µL)
| Vol H2O (µL)
| n° tube
|
| MP144.3
| L128.3
| 163
| 3.68
| 10.32
| AD1
|
| MP144.4
| L128.4
| 154
| 3.90
| 10.10
| AD2
|
| MP145.6
| L129.6
| 163
| 3.68
| 10.32
| AD3
|
| MP145.7
| L129.7
| 188
| 3.19
| 10.81
| AD4
|
| MP146.1
| L130.1
| 298
| 2.01
| 11.99
| AD5
|
| MP146.2
| L130.2
| 142
| 4.23
| 9.77
| AD6
|
- Protocol (see # 3) Experiments done by QIAcube
| Name
| Ligation
| Description
| Biobricks
|
| MP147.1
| L100.1
| rbs TetR - ECFP
D110 (BV) - D130 (BI)
|      
|
| MP147.2
| L100.2
|
| MP147.3
| L100.3
|
| MP148.1
| L101.1
| rbs TetR - GFP tripart
D110 (BV) - D131 (BI)
|      
|
| MP148.2
| L101.2
|
| MP148.3
| L101.3
|
| MP149.1
| L114.1
| AracpBAD - gfp tripart
D126 (BV) - D131 (BI)
|     
|
| MP149.2
| L114.2
|
| MP149.3
| L114.3
|
| MP150.1
| L120.1
| tetR repressible promoter - ECFP
D106 (BV) - D130 (BI)
|     
|
| MP150.2
| L120.2
|
| MP150.3
| L120.3
|
| MP151.1
| L122.1
| RBS-lasI - ECFP
D107 (BV) - D130 (BI)
|      
|
| MP152.1
| L123.1
| RBS lasI - gfp tripart
D107 (BV) - D131 (BI)
|      
|
| MP151.2
| L123.2
|
| MP152.3
| L123.3
|
| MP153.1
| L126.1
| Strongest RBS (1)- LasR activator (+LVA)
D102 (BV) - D114 (BI)
|  
|
| MP153.2
| L126.2
|
| MP153.3
| L126.3
|
| MP154.1
| L132.1
| flhDC
D142(FV) - D139(FI)
| 
|
| MP154.2
| L132.2
|
| MP154.3
| L132.3
|
| MP155.1
| L133.1
| OmpR*
D142(FV) - D140(FI)
| 
|
| MP156.1
| L134.1
| EnvZ*
D142(FV) - D141(FI)
| 
|
| MP156.2
| L134.2
|
| MP156.3
| L134.3
|
| MP157.1
| L138.1
| gfp generator (E0240)
D137(FV) - D138(FI)
|    
|
Glycerol Stocks
| Strain
| Ligation
| Biobricks
| Description
|
| S146.1
| L100.1
| rbs TetR - ECFP
D110 (BV) - D130 (BI)
|      
|
| S146.2
| L100.2
|
| S146.3
| L100.3
|
| S147.1
| L101.1
| rbs TetR - GFP tripart
D110 (BV) - D131 (BI)
|      
|
| S147.2
| L101.2
|
| S147.3
| L101.3
|
| S148.1
| L114.1
| AracpBAD - gfp tripart
D126 (BV) - D131 (BI)
|  
|
| S148.2
| L114.2
|
| S148.3
| L114.3
|
| S149.1
| L120.1
| tetR repressible promoter - ECFP
D106 (BV) - D130 (BI)
|     
|
| S149.2
| L120.2
|
| S149.3
| L120.3
|
| S150.1
| L122.1
| RBS-lasI - ECFP
D107 (BV) - D130 (BI)
|      
|
| S151.1
| L123.1
| RBS lasI - ECFP
D107 (BV) - D131 (BI)
|     
|
| S151.2
| L123.2
|
| S151.3
| L123.3
|
| S152.1
| L126.1
| Strongest RBS (1)- LasR activator (+LVA)
D102 (BV) - D114 (BI)
|  
|
| S152.2
| L126.2
|
| S152.3
| L126.3
|
- sample used: yesterday PCR product of L130.8 (~40 µL)
- protocol used: Qiagen PCR purification kit
- use of buffer QG (Gel Extraction kit) instead of buffer PBI (PCR purification kit)
- elution in 30 µL of buffer EB
- 30 µL of purified product + 12 µL of loading blue
- electrophoresis, 10 µL loaded
=> see Gel 1, well n° 8
==> results: PCR products can be purified using the Qiaquick Gel Extraction kit. We just have to replace the buffer PBI by the buffer QG!
PCR screening
Electrophoresis Setting
4 more transformants of L130 (pFlhB into J61002) are screened by PCR.
- PCR screening programm; elogation time: 1 min 30
- template: colonies from the transformation plate
- positive control: S142 (J61002)
- negative control: no template
- primers: O18 and O19
Results of Electrophoresis
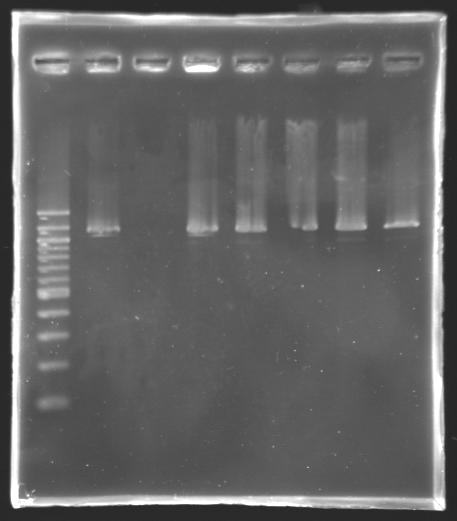 Gel 1: PCR screening ( 2-7) & PCR product purification using the Qiaquick Gel Extraction kit ( 8) - 1% agarose gel
- 10 µL loaded
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
|
| sample
| 100 bp DNA ladder
| positive control
| negative control
| L130.3
| L130.4
| L130.5
| L130.6
| PCR product (L130.8) purified by the Qiaquick Gel Extraction kit
|
| red fluorescence
|
| strain a little bit pink
| not concerned
| no
| no
| no
| no
| not concerned
|
| expected size
| 1,161 kb
| 0 kb
| 1,338 kb
|
| measured size
| 1,2 kb
| 0 kb
| 1,2 kb
| 1,2 kb
| 1,2 kb
| 1,2 kb
|
==> The L130 transformants analysed are not correct.
Transformation of the ligation we did yesterday
We transformed L 139, L140, L141 and L142 following the standard protocol using Invitrogen's TOP 10 chemically competent cells. The positive control is a transformation with pUC19 and the negative control has no plasmid.
|
 "
"

