Team:Hawaii/Notebook/2008-08- 6
From 2008.igem.org
(Difference between revisions)
(→Discussion) |
(→Sequencing) |
||
| (3 intermediate revisions not shown) | |||
| Line 81: | Line 81: | ||
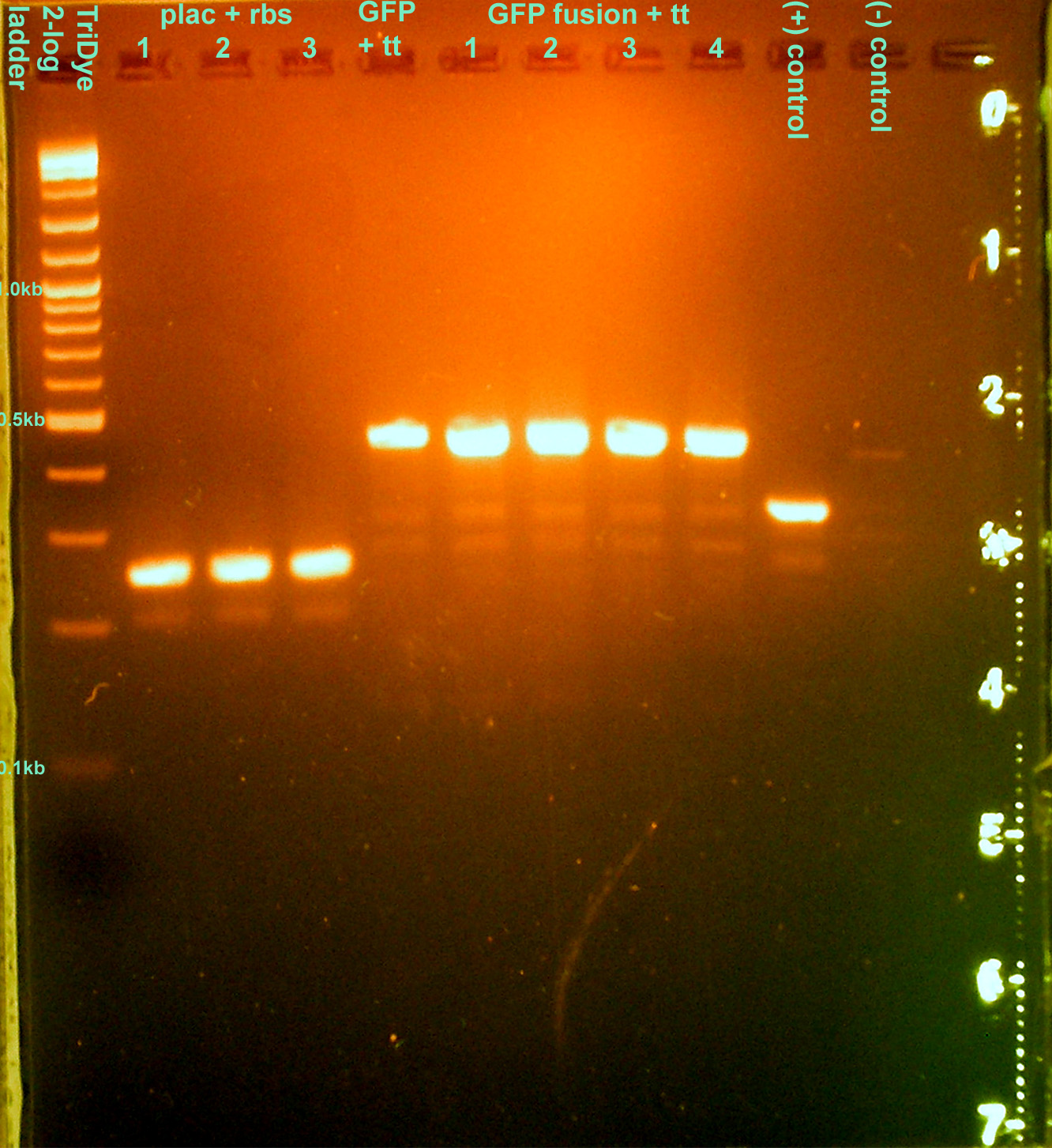
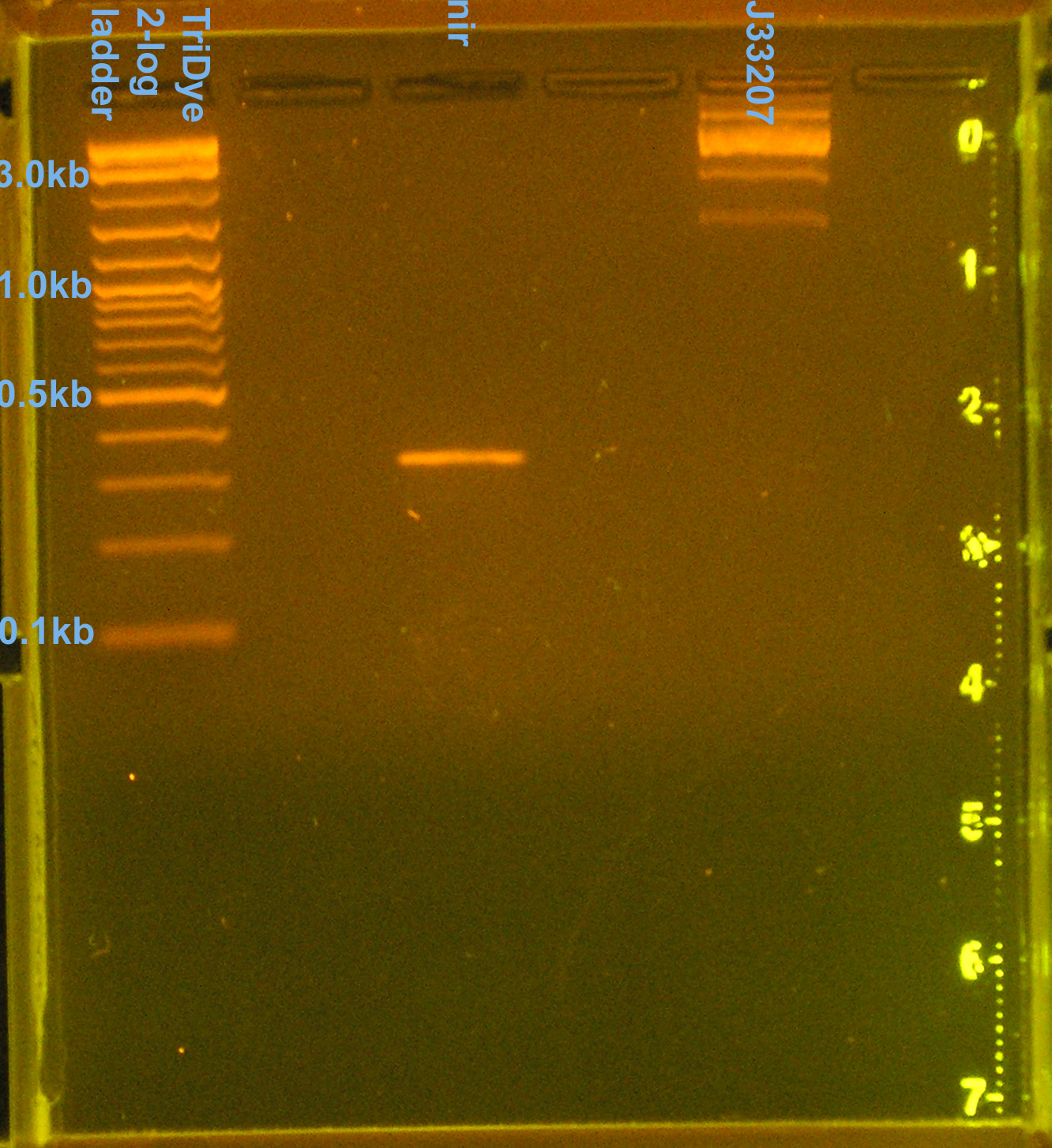
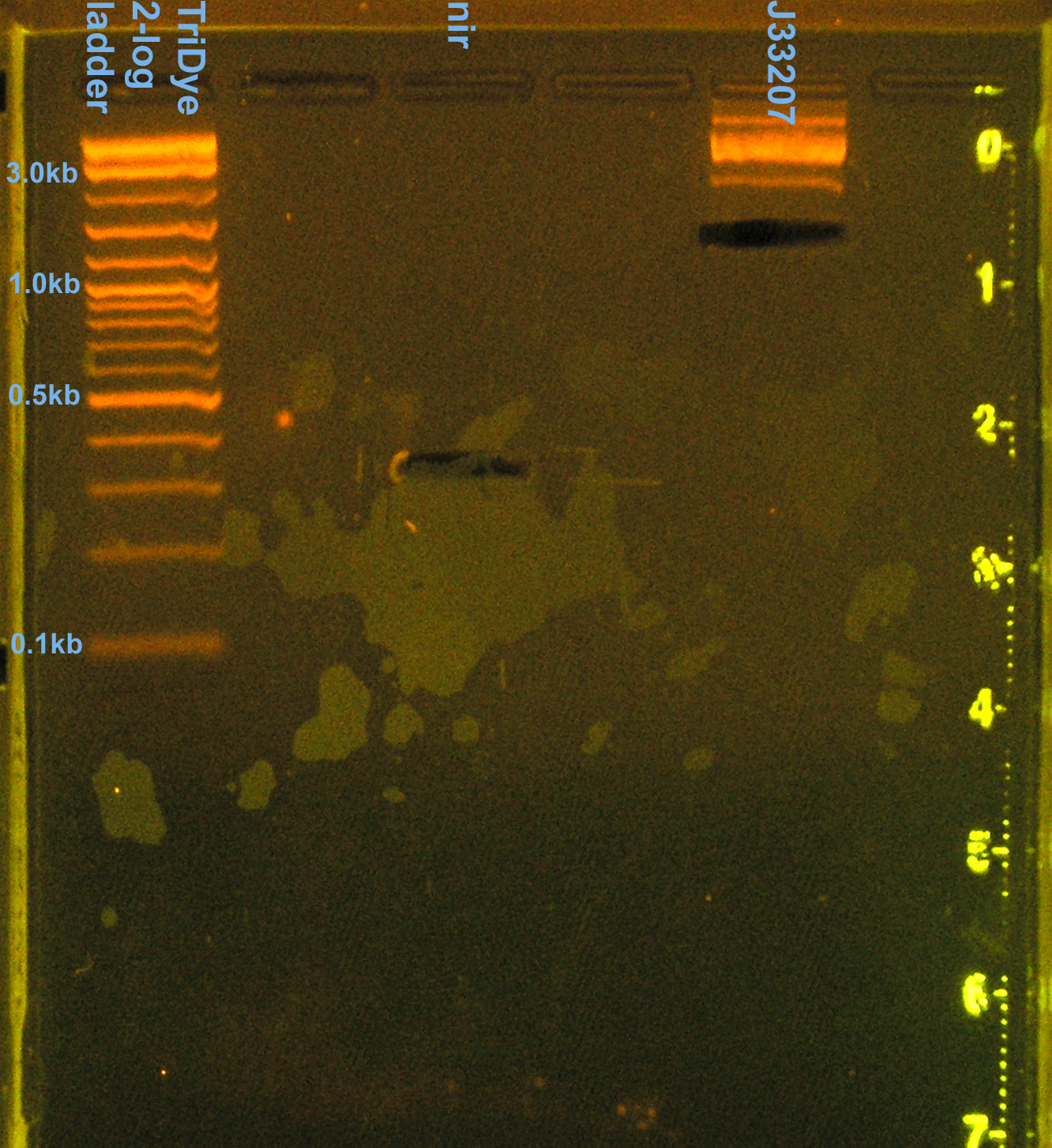
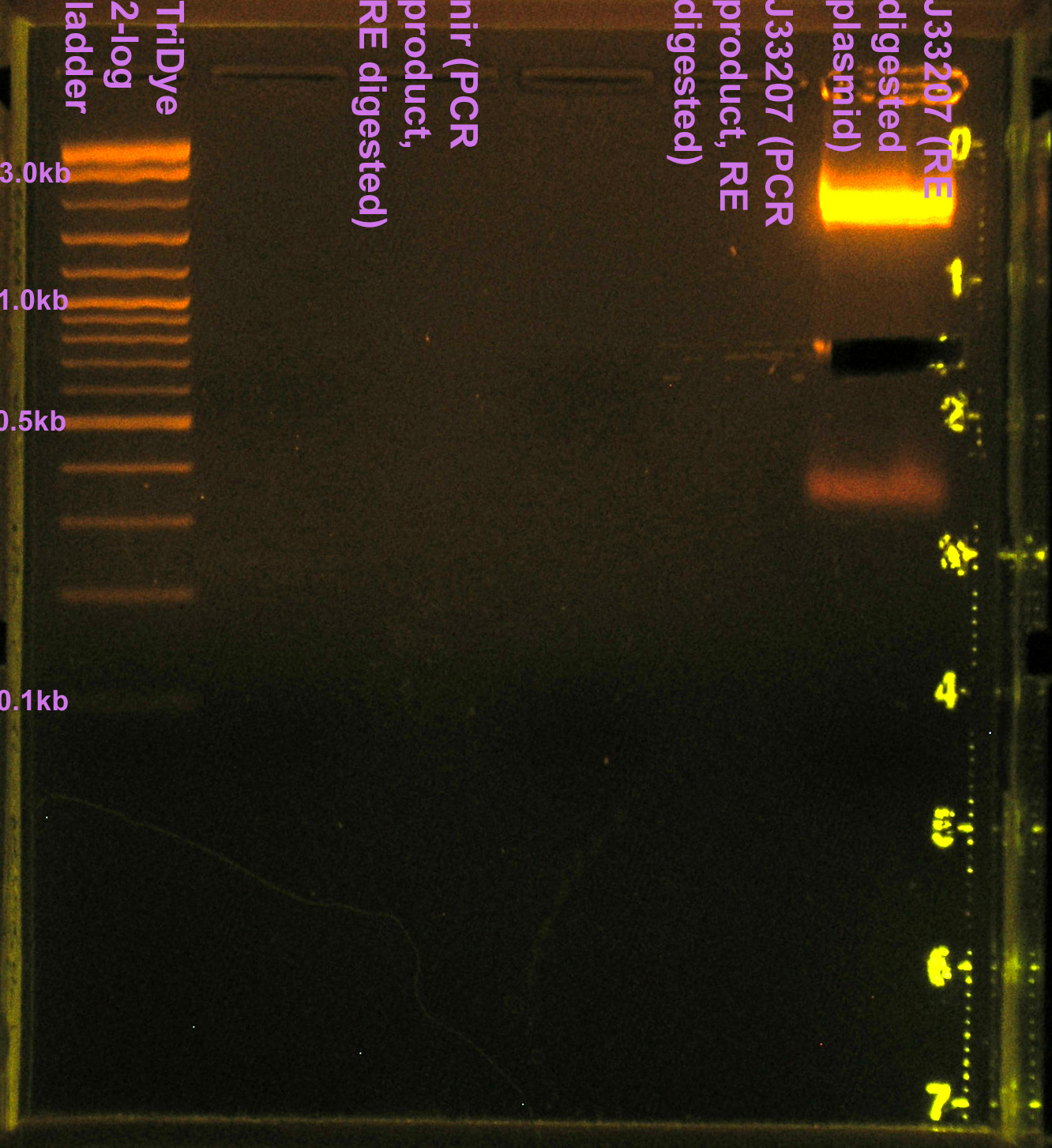
[[Image: 080608nirJ33207pcr.jpg|left|thumb|200px|EtBr stained 2% agarose gel ran at 60V for 100 min. Seven microliters of the PCR reactions were loaded into each lane.]][[Image: 080608nirJ33207pcrcutout.jpg|left|thumb|200px|nir (300bp) and (incorrect) J33207 (1.5kb) bands excised from an EtBr stained 2% agarose gel ran at 60V for 100 min.]][[Image: 080608nirJ33207RE.jpg|left|thumb|200px|EtBr stained 2% agarose gel ran at 72V for 100 min. Fifty microliters of the restriction digests were loaded into each well. ]][[Image: 080608nirJ33207REcut.jpg|left|thumb|200px|J33207 plasmid prep band at ~850bp excised from an EtBr stained 2% agarose gel ran at 72V for 100 min.]] | [[Image: 080608nirJ33207pcr.jpg|left|thumb|200px|EtBr stained 2% agarose gel ran at 60V for 100 min. Seven microliters of the PCR reactions were loaded into each lane.]][[Image: 080608nirJ33207pcrcutout.jpg|left|thumb|200px|nir (300bp) and (incorrect) J33207 (1.5kb) bands excised from an EtBr stained 2% agarose gel ran at 60V for 100 min.]][[Image: 080608nirJ33207RE.jpg|left|thumb|200px|EtBr stained 2% agarose gel ran at 72V for 100 min. Fifty microliters of the restriction digests were loaded into each well. ]][[Image: 080608nirJ33207REcut.jpg|left|thumb|200px|J33207 plasmid prep band at ~850bp excised from an EtBr stained 2% agarose gel ran at 72V for 100 min.]] | ||
:<strong>Krystle</strong> | :<strong>Krystle</strong> | ||
| - | :* Used 5 μl ligation reaction to transform 50 μl DB3.1 cells | + | :* Ligated using quick ligase buffer: |
| + | :** 3 μl gfpf with 1 μl B0015 (tt) | ||
| + | :** 3 μl gfp with 1 μl B0015 (tt) | ||
| + | :** 3 μl nir with 1 μl B0030 (rbs) | ||
| + | :** 3 μl I14032 with 1 μl B0030 (rbs) | ||
| + | |||
| + | :* Used 5 μl ligation reaction to transform 50 μl DB3.1 cells with the the 5 ligations mentioned above | ||
===Made LB+amp<sub>100</sub> plates=== | ===Made LB+amp<sub>100</sub> plates=== | ||
| - | :<strong> Grace and | + | :<strong> Grace and Margaret</strong> |
===Plasmid Prep=== | ===Plasmid Prep=== | ||
| Line 91: | Line 97: | ||
:*pSMC121 was plasmid prepped today | :*pSMC121 was plasmid prepped today | ||
| + | :<strong> Krystle </strong> | ||
| + | :*Started 200 ml preps of GFPf, nir, and pRL1383aM | ||
===PCR=== | ===PCR=== | ||
| Line 102: | Line 110: | ||
:* Reply from CORE Hawaii | :* Reply from CORE Hawaii | ||
| + | ::* They will resequence slr1, slr2, BB-pRL1383a for free due to potential mix up of samples and poor quality reads | ||
| + | ::* For samples that were overloaded when 20ng/100bp sequencing guideline used, try 5 ng/100bp instead. | ||
= Discussion = | = Discussion = | ||
Latest revision as of 16:41, 9 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Checked transformants from yesterday
- Grace
| Construct | Colony forming units |
|---|---|
| I14032 (plac) + B0030 (rbs) | 3 |
| pnir + B003 (rbs) | 0 |
| E0040 (GFP) + B0015 (tt) | 1 |
| GFPf + B0015 (tt) | 2 + 2 clusters of colonies |
- Colony PCR'd transformants
- 30 cycles, anneal at 62C, extend for 1 min.
- Ran on EtBr stained 2.0% agarose gel at 95V for 1 hour
- None of the transformations were successful :o(
Determined DNA concentrations of purified RE'd PCR products from 8/4
- Grace
| DNA sample | Concentration |
|---|---|
| nir | 17.0 ng/μl |
| slr1 | 31.3 ng/μl |
| slr2 | 11.8 ng/μl |
| pilA | 13.0 ng/μl |
| GFP | 12.0 ng/μl |
| GFPf | 12.2 ng/μl |
| E0240 | 8.6 ng/μl |
| I14032 | 16.8 ng/μl |
Prepared PCR'd nir and J33207 (from yesterday) for transformation/ligation
- Grace
- Ran PCR products on EtBr stained 2.0% agarose gel at 60V for 100 min.
- nir band at 330bp confirmed
- J33207 = 4 bands (1.5kb, 2.5kb, 3.2kb, 10kb), none of which are correct
- [http://www.partsregistry.org partsregistry] says J33207 DNA is inconsistent
- Extracted nir and 1.5kb J33207 band from gel
- RE digested in 50 μl rxns with EcoRI and SpeI in NEBuffer 2
- Larger rxn volume may improve digest efficiency
- Incubated at 37C for 2.5 hours
- RE digested 10 μl J33207 plasmid prep with EcoRI and SpeI in NEBuffer 2
- To confirm plasmid prep; if good, will use for ligation rxn
- Incubated at 37C for 2 hours
- Ran RE digests on EtBr stained 2.0% agarose gel at 72V for 90 min.
- No visible bands for nir and J33207 RE digest
- We lost a LOT of DNA in the gel purification.
- Band for J33207 plasmid prep RE digest should be ~650bp
- Band at ~850bp = VF2-VR = RE didn't cut???
- Extracted J33207 band (~850) from gel
- Ligated 4.5 μl J33207 with 1.5 μl B0015 (tt)
- Krystle
- Ligated using quick ligase buffer:
- 3 μl gfpf with 1 μl B0015 (tt)
- 3 μl gfp with 1 μl B0015 (tt)
- 3 μl nir with 1 μl B0030 (rbs)
- 3 μl I14032 with 1 μl B0030 (rbs)
- Ligated using quick ligase buffer:
- Used 5 μl ligation reaction to transform 50 μl DB3.1 cells with the the 5 ligations mentioned above
Made LB+amp100 plates
- Grace and Margaret
Plasmid Prep
- Margaret
- pSMC121 was plasmid prepped today
- Krystle
- Started 200 ml preps of GFPf, nir, and pRL1383aM
PCR
- Margaret
- made large quantities of aadA, rep, and oriV. Please refer to The experiment write-up for more details.
Drylab work
Sequencing
- Grace
- Reply from CORE Hawaii
- They will resequence slr1, slr2, BB-pRL1383a for free due to potential mix up of samples and poor quality reads
- For samples that were overloaded when 20ng/100bp sequencing guideline used, try 5 ng/100bp instead.
Discussion
- We're low on small nitrile gloves and Qiagen spin columns. Need to order more. NW
- Be careful when pouring agar plates. Bubbles suck!
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"