Team:ESBS-Strasbourg/measurements
From 2008.igem.org
(Difference between revisions)
(→Effect of the repressor) |
|||
| (20 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
<div id="header">{{Template:Team:ESBS-Strasbourg/Templates/Header}}</div> | <div id="header">{{Template:Team:ESBS-Strasbourg/Templates/Header}}</div> | ||
<div id="maincontent" style="margin-top:170px;"> | <div id="maincontent" style="margin-top:170px;"> | ||
| - | |||
| - | |||
| - | |||
__NOTOC__ | __NOTOC__ | ||
| + | =Measurements= | ||
| + | <br> | ||
| + | ==Introductory Explanations== | ||
| + | ===General=== | ||
| + | * The promoter consists of a repeat of the operator (binding sequence of the DB) and the minimal Promoter (containing TATA box and the Kozak sequence) | ||
| + | ===Abreviations=== | ||
| + | * BD: DNA-binding domain of either LexA, LacI, CI or TetR | ||
| + | |||
| - | == | + | ==Line 1: Activity and functionality of the single activator constructs== |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
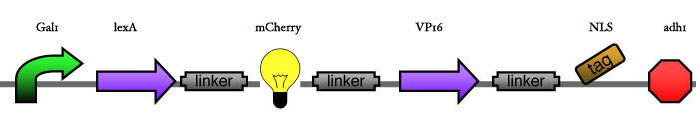
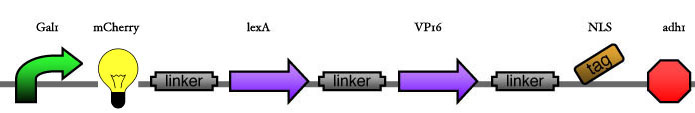
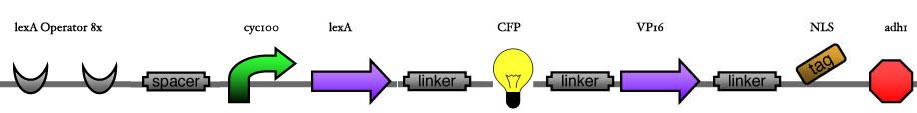
Here we are going to test two things: | Here we are going to test two things: | ||
* The functionality of the inducer construct in regard to the position of the XFP. This will first be tested for one construct and then later on be transfered to the others. '''The thing to measure''' is the amount of CFP under same inducer conditions for the three constructs. | * The functionality of the inducer construct in regard to the position of the XFP. This will first be tested for one construct and then later on be transfered to the others. '''The thing to measure''' is the amount of CFP under same inducer conditions for the three constructs. | ||
* The strength of the promoters for the different DNA Binding Proteins. Therefore we will induce our system with different time steps and measure the outcome of the CFP. Then we'll try to find couples of DBP with similar activities/Kds by changing the number of operons. '''The thing to measure''' is the ratio between YFP and CFP under different inducer conditions and different operon numbers and the total amount of CFP.<br> | * The strength of the promoters for the different DNA Binding Proteins. Therefore we will induce our system with different time steps and measure the outcome of the CFP. Then we'll try to find couples of DBP with similar activities/Kds by changing the number of operons. '''The thing to measure''' is the ratio between YFP and CFP under different inducer conditions and different operon numbers and the total amount of CFP.<br> | ||
| - | < | + | [[Image:ESBS-tester11.JPG]]<br> |
| - | [[Image: | + | [[Image:ESBS-tester12.JPG]]<br> |
| + | [[Image:ESBS-tester13.JPG]]<br> | ||
| + | |||
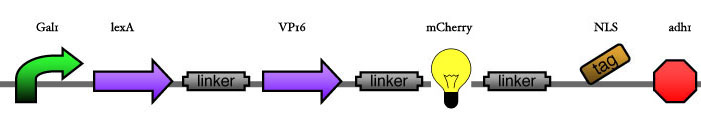
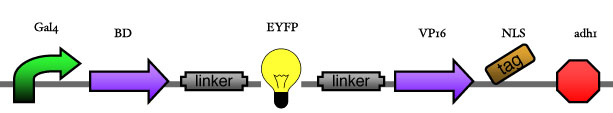
<big> '''1.2 Reporter construct''' </big><br> | <big> '''1.2 Reporter construct''' </big><br> | ||
| - | [[Image: | + | [[Image:ESBS-tester14.JPG]]<br> |
| - | ==Stability of the self activator== | + | |
| + | ==Line 2: Stability of the self activator== | ||
Here we are going to test if the self activation of the constructs is sufficiently strong to keep the signal through several cell divisions. '''The thing to measure''' is the CFP amount after induction and after several (~10) cell divisions.<br> | Here we are going to test if the self activation of the constructs is sufficiently strong to keep the signal through several cell divisions. '''The thing to measure''' is the CFP amount after induction and after several (~10) cell divisions.<br> | ||
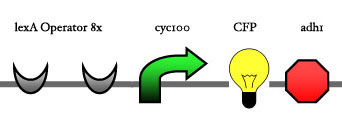
<big> '''2.1 Inducer''' </big><br><br> | <big> '''2.1 Inducer''' </big><br><br> | ||
| - | + | One of the constructs from 1.1 is choosen.<br><br> | |
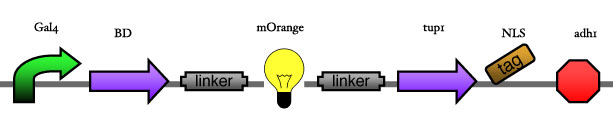
<big> '''2.2 Reporter construct''' </big><br><br> | <big> '''2.2 Reporter construct''' </big><br><br> | ||
| - | [[Image: | + | [[Image:ESBS-tester21.JPG]]<br> |
| - | ==Effect of the repressor== | + | |
| + | ==Line 3: Effect of the repressor== | ||
Here we can measure two things: | Here we can measure two things: | ||
* the efficiency of the repressor. '''The thing to measure''' is the CFP amount.<br> | * the efficiency of the repressor. '''The thing to measure''' is the CFP amount.<br> | ||
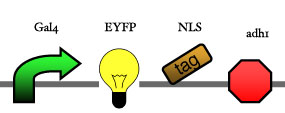
<big> '''3.1 Inducer''' </big><br> | <big> '''3.1 Inducer''' </big><br> | ||
| - | [[Image: | + | [[Image:ESBS-tester31.JPG]]<br> |
| + | [[Image:ESBS-tester32.JPG]]<br> | ||
<big> '''3.1 Reporter construct''' </big><br><br> | <big> '''3.1 Reporter construct''' </big><br><br> | ||
| - | + | [[Image:ESBS-tester14.JPG]]<br> | |
| - | ==Tag-testing== | + | ==Line 4: Tag-testing== |
Here first of all the efficiency of our tags are tested with XFP before we can test out at which position to put them into the BD-VP16-XFP-NLS-PEST-APC construct. '''The thing to measure''' is the development of the XFP.<br> | Here first of all the efficiency of our tags are tested with XFP before we can test out at which position to put them into the BD-VP16-XFP-NLS-PEST-APC construct. '''The thing to measure''' is the development of the XFP.<br> | ||
| - | [[Image: | + | [[Image:ESBS-tester41.JPG]]<br> |
| + | [[Image:ESBS-tester42.JPG]]<br> | ||
| + | [[Image:ESBS-tester43.JPG]]<br> | ||
| + | [[Image:ESBS-tester44.JPG]]<br> | ||
<br> | <br> | ||
| - | |||
Latest revision as of 21:11, 29 October 2008
Measurements
Introductory Explanations
General
- The promoter consists of a repeat of the operator (binding sequence of the DB) and the minimal Promoter (containing TATA box and the Kozak sequence)
Abreviations
- BD: DNA-binding domain of either LexA, LacI, CI or TetR
Line 1: Activity and functionality of the single activator constructs
Here we are going to test two things:
- The functionality of the inducer construct in regard to the position of the XFP. This will first be tested for one construct and then later on be transfered to the others. The thing to measure is the amount of CFP under same inducer conditions for the three constructs.
- The strength of the promoters for the different DNA Binding Proteins. Therefore we will induce our system with different time steps and measure the outcome of the CFP. Then we'll try to find couples of DBP with similar activities/Kds by changing the number of operons. The thing to measure is the ratio between YFP and CFP under different inducer conditions and different operon numbers and the total amount of CFP.
Line 2: Stability of the self activator
Here we are going to test if the self activation of the constructs is sufficiently strong to keep the signal through several cell divisions. The thing to measure is the CFP amount after induction and after several (~10) cell divisions.
2.1 Inducer
One of the constructs from 1.1 is choosen.
2.2 Reporter construct
Line 3: Effect of the repressor
Here we can measure two things:
- the efficiency of the repressor. The thing to measure is the CFP amount.
3.1 Inducer
3.1 Reporter construct
Line 4: Tag-testing
Here first of all the efficiency of our tags are tested with XFP before we can test out at which position to put them into the BD-VP16-XFP-NLS-PEST-APC construct. The thing to measure is the development of the XFP.
 "
"