Team:KULeuven/Model/Inverter
From 2008.igem.org
m (→Parameters) |
m (→Describing the system) |
||
| (10 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{:Team:KULeuven/Tools/Styling}} | ||
| + | {{:Team:KULeuven/Tools/Scripting}} | ||
{{:Team:KULeuven/Tools/Header}} | {{:Team:KULeuven/Tools/Header}} | ||
| - | + | [[Image:pictogram_inverter.png|120px|right]] | |
| + | |||
==Invertimer== | ==Invertimer== | ||
=== Position in the system === | === Position in the system === | ||
| - | The | + | The InverTimer subsystem receives its input from the filter, T7. The InverTimer's function is to '''produce''' HSL when '''no''' input is present, so a low T7 input gives rise to a high HSL output and vice versa. The production of HSL means that the cell will start a timer that eventually will be used in the celldeath-subsystem to produce ccdB. In this way the cell will die off if no desease remains present. |
=== Describing the system === | === Describing the system === | ||
| - | see also: [https://2008.igem.org/Team:KULeuven/Project/Inverter Project: | + | see also: [https://2008.igem.org/Team:KULeuven/Project/Inverter Project:InverTimer] |
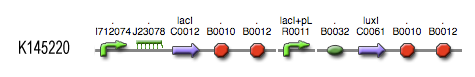
[[Image:Inverter_BioBrick.jpg|center]] | [[Image:Inverter_BioBrick.jpg|center]] | ||
| Line 18: | Line 21: | ||
<body> | <body> | ||
<p> | <p> | ||
| - | <a href="https://static.igem.org/mediawiki/2008/ | + | <a href="https://static.igem.org/mediawiki/2008/3/35/InverterODE.pdf"> |
<img border="0" src="https://2008.igem.org/wiki/skins/common/images/icons/fileicon-pdf.png" width="65" height="60"> | <img border="0" src="https://2008.igem.org/wiki/skins/common/images/icons/fileicon-pdf.png" width="65" height="60"> | ||
</a> | </a> | ||
| Line 165: | Line 168: | ||
| k<sub>transl LacI</sub> | | k<sub>transl LacI</sub> | ||
| 0.167 s<sup>-1</sup> | | 0.167 s<sup>-1</sup> | ||
| - | | lock defined translation rate for LacI | + | | estimate: lock defined translation rate for LacI |
| [https://2008.igem.org/Team:KULeuven/Model/Inverter#References [2<html>]</html>] | | [https://2008.igem.org/Team:KULeuven/Model/Inverter#References [2<html>]</html>] | ||
|} | |} | ||
| Line 182: | Line 185: | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| - | ! Time span | + | !width="80"| Time span |
| - | ! Input (TetR) | + | !width="80"| Input (TetR) |
| - | ! Results | + | !width="720"| Results |
|- | |- | ||
| A | | A | ||
| - | | 0.0125 | + | | 0.0125 s<sup>-1</sup> |
| The amount LacI increases from state zero to state one because both mRNA_RIBOKEY and pT7_tag are present. This results in a repression of LuxI which decreases to zero: the input signal (TetR) is inverted. | | The amount LacI increases from state zero to state one because both mRNA_RIBOKEY and pT7_tag are present. This results in a repression of LuxI which decreases to zero: the input signal (TetR) is inverted. | ||
|- | |- | ||
| B | | B | ||
| - | | 5E-5 | + | | 5E-5 s<sup>-1</sup> |
| The amount LacI decreases back to state zero. The amount LuxI remains the same (state zero). | | The amount LacI decreases back to state zero. The amount LuxI remains the same (state zero). | ||
|- | |- | ||
| C | | C | ||
| - | | 5E-5 | + | | 5E-5 s<sup>-1</sup> |
| LuxI changes from state zero to state one. Time span B and C form together the transient behaviour of the inverter when the input signal changes from one to zero. | | LuxI changes from state zero to state one. Time span B and C form together the transient behaviour of the inverter when the input signal changes from one to zero. | ||
|- | |- | ||
| D | | D | ||
| - | | 5E-5 | + | | 5E-5 s<sup>-1</sup> |
| LuxI remains in state one: the input signal is once again inverted. | | LuxI remains in state one: the input signal is once again inverted. | ||
|- | |- | ||
| E | | E | ||
| - | | 0.0125 | + | | 0.0125 s<sup>-1</sup> |
| A short pulse of 1000 seconds has a influence a steep decrease of LuxI. | | A short pulse of 1000 seconds has a influence a steep decrease of LuxI. | ||
|- | |- | ||
| F & G | | F & G | ||
| - | | 5E-5 | + | | 5E-5 s<sup>-1</sup> |
| During time span F and G, LuxI decreases further for a while and increases back to state one. | | During time span F and G, LuxI decreases further for a while and increases back to state one. | ||
|- | |- | ||
| H | | H | ||
| - | | 5E-5 | + | | 5E-5 s<sup>-1</sup> |
| LuxI is back in state one. | | LuxI is back in state one. | ||
|} | |} | ||
The simulation shows a working inverter (left figure). A small disadvantage is the transient behaviour of the inverter: a small pulse of 1000 seconds results in a transient behaviour of +- 30000 seconds. Also for a long pulse (10000 seconds) is a long transient behaviour noticeable (40000 seconds). The effect of the inverter on the timer aspect is visuable in the right figure: a long pulse ( from 10000 till 11000) resets the timer (HSL drecreases till zero). After this pulse and the transient behaviour of the inverter, the timer restarts counting. The short pulse (from 200000 till 201000 seconds) only partially resets the timer. | The simulation shows a working inverter (left figure). A small disadvantage is the transient behaviour of the inverter: a small pulse of 1000 seconds results in a transient behaviour of +- 30000 seconds. Also for a long pulse (10000 seconds) is a long transient behaviour noticeable (40000 seconds). The effect of the inverter on the timer aspect is visuable in the right figure: a long pulse ( from 10000 till 11000) resets the timer (HSL drecreases till zero). After this pulse and the transient behaviour of the inverter, the timer restarts counting. The short pulse (from 200000 till 201000 seconds) only partially resets the timer. | ||
| + | |||
| + | All graphs have amounts (number of molecules in the cell) plotted vs time, measured in seconds. | ||
<html> | <html> | ||
| Line 224: | Line 229: | ||
<img src="https://static.igem.org/mediawiki/2008/2/27/HSL.png" style="float: left; width: 600px; height: 500px; margin: 0 5px;" /> | <img src="https://static.igem.org/mediawiki/2008/2/27/HSL.png" style="float: left; width: 600px; height: 500px; margin: 0 5px;" /> | ||
</div></div></div></html> | </div></div></div></html> | ||
| - | |||
| - | |||
| - | |||
| - | |||
=== References === | === References === | ||
| Line 243: | Line 244: | ||
<tr><td colspan="2"> </td></tr> | <tr><td colspan="2"> </td></tr> | ||
| - | <tr style="vertical-align:top;"><td>[3]</td><td style="padding-left:4pt;">“ETHZ/Parameters - IGEM07”; | + | <tr style="vertical-align:top;"><td>[3]</td><td style="padding-left:4pt;">“ETHZ/Parameters - IGEM07”; https://2007.igem.org/ETHZ/Parameters.</td></tr> |
<tr><td colspan="2"> </td></tr> | <tr><td colspan="2"> </td></tr> | ||
<tr style="vertical-align:top;"><td>[4]</td><td style="padding-left:4pt;">“Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein,” Sep. 1996; http://www.pnas.org/content/93/18/9505.</td></tr> | <tr style="vertical-align:top;"><td>[4]</td><td style="padding-left:4pt;">“Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein,” Sep. 1996; http://www.pnas.org/content/93/18/9505.</td></tr> | ||
Latest revision as of 23:20, 29 October 2008
Contents |
Invertimer
Position in the system
The InverTimer subsystem receives its input from the filter, T7. The InverTimer's function is to produce HSL when no input is present, so a low T7 input gives rise to a high HSL output and vice versa. The production of HSL means that the cell will start a timer that eventually will be used in the celldeath-subsystem to produce ccdB. In this way the cell will die off if no desease remains present.
Describing the system
see also: Project:InverTimer
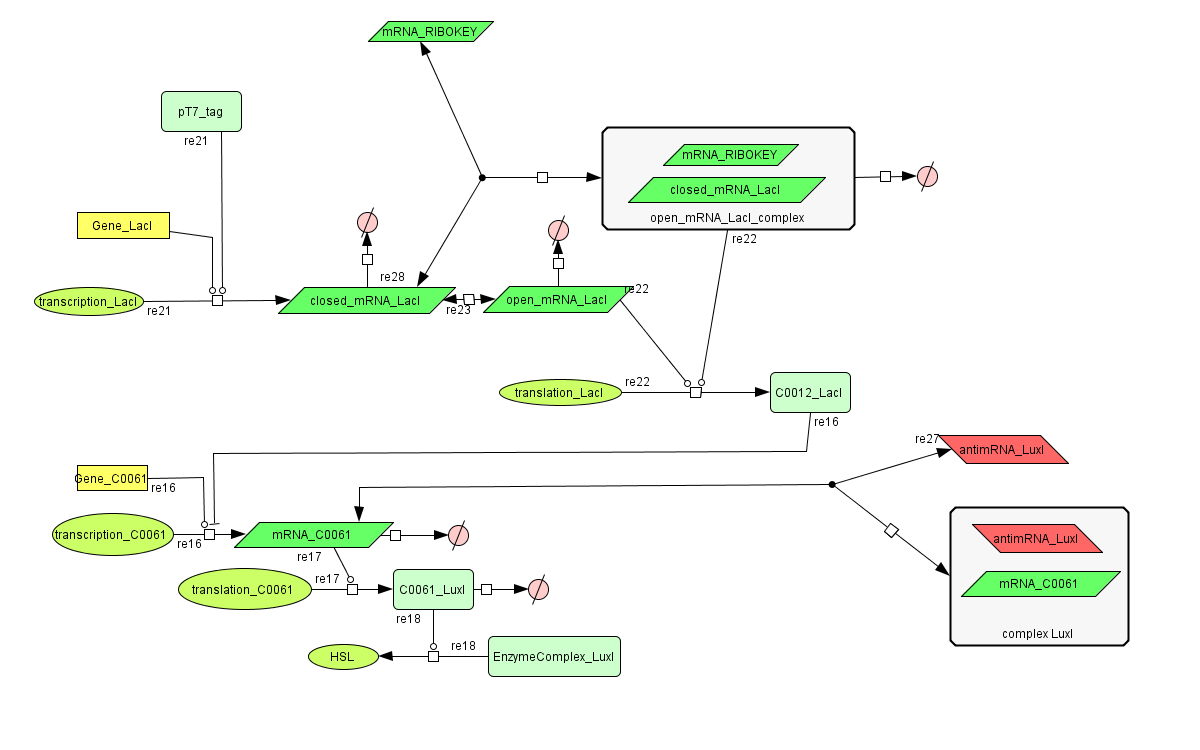
ODE's
Parameters
| Name | Value | Comments | Reference |
|---|---|---|---|
| Degradation Rates | |||
| dLuxI | dLVA = 2.814E-4 s-1 | LVA-tag reduces lifetime to 40 minutes | [2] [7] |
| dRNA_LuxI | 0.0025 s-1 | [5] | |
| dLuxI_antimRNA | 0.0045303737 s-1 | estimate: because this RNA isn't translated, it degrades faster | [5] |
| dLacI | dLVA = 2.814E-4 s-1 | LVA-tag reduces lifetime to 40 minutes | [2] [7] |
| dclosed mRNA LacI | 0.0046209812 s-1 | estimate: because this mRNA isn't translated, it degrades faster | [5] |
| dopen mRNA LacI | 0.0023104906 s-1 | [5] | |
| dopen mRNA LacI complex | 0.0023104906 s-1 | [5] | |
| dHSL | 1.02E-6 s-1 | very stable in the medium, lifetime around 185h | [11] |
| LuxI catalysis | |||
| kcat | 0.0166666667 s-1 | Estimated to be about 90% of Vmax in LB medium. | [4] |
| T7 Transcription | |||
| KT7 | 421 | dissociation constant, recalculated to remove units | [10] |
| kmax | 0.044 s-1 | maximal T7 transcription rate | [10] |
| Key-Lock constants | |||
| Keq 1 | 0,015 [M] | between closed and open T7 mRNA, modeled for competition, experimental | [2] |
| Keq 2 | 0.0212 [M] | between closed T7 mRNA and key unlocked mRNA complex, modeled for competition, experimental | [2] |
| kdis2 | 0.00416 s-1 | derived from experimental values | [2] |
| kcomplex2 | 0.00237 s-1 | derived from experimental values | [2] |
| kclosed | 500 s-1 | derived from experimental values | [2] |
| kopen | 7.5 s-1 | derived from experimental values | [2] |
| LacI repression | |||
| KLacI | 1.0E-10 M-1 | Dissociation constant | [3] |
| nLacI | 2.0 | Hill coefficient for LacI | [3] |
| k_trans_LacI | 0.0025 s-1 | Estimated maximal transcription rate from R0011 | [9] |
| Antisense LuxI | |||
| k_complex3 | 0.00237 s-1 | rate constant for formation of asRNA - LuxI mRNA duplex | [3] |
| KmRNA_LuxI:antisense_mRNA | 4.22E14 | Complex of LuxI mRNA with antisense mRNA | [1] |
| Translation Rates | |||
| ktransl LuxI | 0.167 s-1 | translation rate for B0032 RBS (0.3 relative efficiency) | [8] |
| ktransl LacI | 0.167 s-1 | estimate: lock defined translation rate for LacI | [2] |
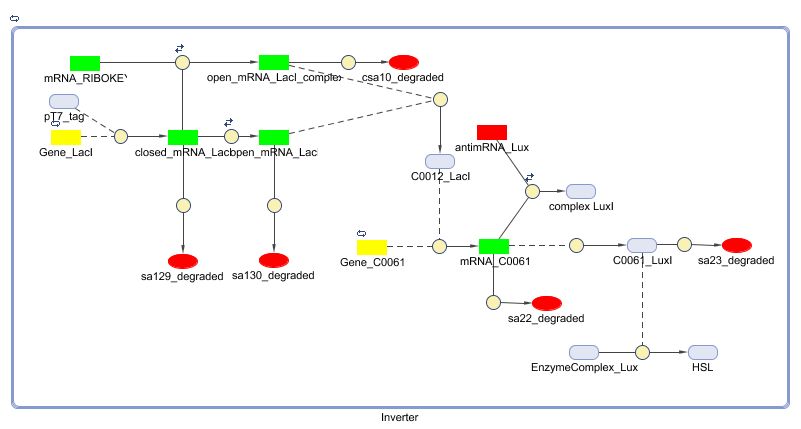
Models
CellDesigner (SBML file)
Matlab (SBML file)
Simulations
| Time span | Input (TetR) | Results |
|---|---|---|
| A | 0.0125 s-1 | The amount LacI increases from state zero to state one because both mRNA_RIBOKEY and pT7_tag are present. This results in a repression of LuxI which decreases to zero: the input signal (TetR) is inverted. |
| B | 5E-5 s-1 | The amount LacI decreases back to state zero. The amount LuxI remains the same (state zero). |
| C | 5E-5 s-1 | LuxI changes from state zero to state one. Time span B and C form together the transient behaviour of the inverter when the input signal changes from one to zero. |
| D | 5E-5 s-1 | LuxI remains in state one: the input signal is once again inverted. |
| E | 0.0125 s-1 | A short pulse of 1000 seconds has a influence a steep decrease of LuxI. |
| F & G | 5E-5 s-1 | During time span F and G, LuxI decreases further for a while and increases back to state one. |
| H | 5E-5 s-1 | LuxI is back in state one. |
The simulation shows a working inverter (left figure). A small disadvantage is the transient behaviour of the inverter: a small pulse of 1000 seconds results in a transient behaviour of +- 30000 seconds. Also for a long pulse (10000 seconds) is a long transient behaviour noticeable (40000 seconds). The effect of the inverter on the timer aspect is visuable in the right figure: a long pulse ( from 10000 till 11000) resets the timer (HSL drecreases till zero). After this pulse and the transient behaviour of the inverter, the timer restarts counting. The short pulse (from 200000 till 201000 seconds) only partially resets the timer.
All graphs have amounts (number of molecules in the cell) plotted vs time, measured in seconds.


References
| [1] | A. E G H Wagner and R W Simons, “Antisense RNA Control in Bacteria, Phages, and Plasmids,” Nov. 2003; http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.mi.48.100194.003433. |
| [2] | “Berkeley2006-RiboregulatorsMain - IGEM”; http://parts2.mit.edu/wiki/index.php/Berkeley2006-RiboregulatorsMain. |
| [3] | “ETHZ/Parameters - IGEM07”; https://2007.igem.org/ETHZ/Parameters. |
| [4] | “Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein,” Sep. 1996; http://www.pnas.org/content/93/18/9505. |
| [5] | J.A. Bernstein et al., “Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays,” Proceedings of the National Academy of Sciences of the United States of America, vol. 99, Jul. 2002, pp. 9697–9702. |
| [6] | W. Hsieh et al., “Influence of sequence and distance between two operators on interaction with the lac repressor,” J. Biol. Chem., vol. 262, Oct. 1987, pp. 14583-14591. |
| [7] | J.B. Andersen et al., “New Unstable Variants of Green Fluorescent Protein for Studies of Transient Gene Expression in Bacteria,” Applied and Environmental Microbiology, vol. 64, Jun. 1998, pp. 2240–2246. |
| [8] | “Part:BBa B0032 - partsregistry.org”; http://partsregistry.org/Part:BBa_B0032. |
| [9] | “Part:BBa R0011 - partsregistry.org”; http://partsregistry.org/Part:BBa_R0011. |
| [10] | G.M. Skinner et al., “Promoter Binding, Initiation, and Elongation By Bacteriophage T7 RNA Polymerase: A SINGLE-MOLECULE VIEW OF THE TRANSCRIPTION CYCLE,” J. Biol. Chem., vol. 279, Jan. 2004, pp. 3239-3244. |
| [11] | Y. Wang and J.R. Leadbetter, “Rapid Acyl-Homoserine Lactone Quorum Signal Biodegradation in Diverse Soils,” Appl. Environ. Microbiol., vol. 71, Mar. 2005, pp. 1291-1299. |
 "
"