Team:Valencia/Project/Lab work/2 experiments
From 2008.igem.org
| (21 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
<div style=" width:94%; margin: 0 auto;"> | <div style=" width:94%; margin: 0 auto;"> | ||
| - | + | ||
<h2>2.-Demonstration that thermogenin-expressing yeast strains can heat their own broth medium.</h2> | <h2>2.-Demonstration that thermogenin-expressing yeast strains can heat their own broth medium.</h2> | ||
| Line 15: | Line 15: | ||
*UCP- : UCP1 antisense gene with Gal7 promoter. It is used as control strain. | *UCP- : UCP1 antisense gene with Gal7 promoter. It is used as control strain. | ||
*[[Team:Valencia/Parts/UCP175deleted | Gly175Δ]] : UCP1 mutant sequence, Glycine 175 has been deleted. | *[[Team:Valencia/Parts/UCP175deleted | Gly175Δ]] : UCP1 mutant sequence, Glycine 175 has been deleted. | ||
| - | *[[Team:Valencia/Parts/UCP76deleted | Gly76Δ]] : UCP1 mutant sequence, Glycine 76 has been deleted. | + | *[[Team:Valencia/Parts/UCP76deleted | Gly76Δ]] : UCP1 mutant sequence, Glycine 76 has been deleted. These two strains have increased uncoupling activity and do not need fatty acids to be functional. |
| Line 21: | Line 21: | ||
[[Image:Valencia_pYeDP.png|500px]] | [[Image:Valencia_pYeDP.png|500px]] | ||
| - | <h3> Method </h3> | + | <h3> Method of culturing UCP1-producing yeast strains for heat production </h3> |
Each of our experiments entails the following steps: | Each of our experiments entails the following steps: | ||
<ol><li> We prepare a 30ml inoculum for each strains in 100ml Erlenmeyer flasks with [[Team:Valencia/Notebook/Protocols#SD Medium | SD medium]]. We leave it overnight in the 28ºC stove. <br> | <ol><li> We prepare a 30ml inoculum for each strains in 100ml Erlenmeyer flasks with [[Team:Valencia/Notebook/Protocols#SD Medium | SD medium]]. We leave it overnight in the 28ºC stove. <br> | ||
| - | </li><li> We prepare a second inoculum for each strain, this time | + | </li><li> We prepare a second inoculum for each strain, this time 100ml [[Team:Valencia/Notebook/Protocols#SP liquid medium | SP medium]] in 1L Erlenmeyer flasks. The initial O.D. of this second inoculum is adjusted to 0.2. We leave it several hours in the 28ºC stove.<br> |
| - | The objective of this two-step protocol is to be able to start the experiment with a higher O.D. in a medium with little or no glucose. | + | The objective of this two-step protocol is to be able to start the experiment with a higher O.D. in a medium with little or no glucose. In summary: first, we grow our strains in a liquid medium with glucose (SD medium), in which they have a better growth rate. Second, we use SP medium because it has a really small amount of glucose. <br> |
| - | </li><li> We start the experiment in the LCCs with a volume of 100ml | + | </li><li> We start the experiment in the LCCs with a volume of 100ml of [[Team:Valencia/Notebook/Protocols#SP liquid medium | SP medium]], adjusted to O.D. of 0.6. We heat the medium up to aproximately 28ºC inside the LCC.<br> |
| - | </li><li> We add 1% galactose to each of the LCCs containing the four yeast strains. This sugar is the | + | </li><li> We add 1% galactose to each of the LCCs containing the four yeast strains. This sugar is the UCP1 gene Gal7 promoter inductor and it is therefore needed for the production of UCP1. <br> |
</li><li> We monitor the temperature evolution in the LCCs thanks to our [[Team:Valencia/Project/Lab_work | LCC system]]. <br> | </li><li> We monitor the temperature evolution in the LCCs thanks to our [[Team:Valencia/Project/Lab_work | LCC system]]. <br> | ||
</li></ol> | </li></ol> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<h3> Experiment results </h3> | <h3> Experiment results </h3> | ||
{| | {| | ||
| - | |<center>''' | + | |<center><font face="trebuchet ms" style="color:#047DB5" size="4">'''August 15th'''</font></center> |
| - | + | [[Image:Valencia_15-8.png|700px]]<br> | |
| | | | ||
*Volume: 100 ml | *Volume: 100 ml | ||
| Line 59: | Line 46: | ||
*250 rpm and slightly tilt. | *250 rpm and slightly tilt. | ||
*Initial OD: 0,2 | *Initial OD: 0,2 | ||
| - | *O.D measured each one a a half hours during the first 9 hours | + | *O.D measured each one a a half hours during the first 9 hours (We observe each of the moments we opened LCCs for measurements in the graph). |
*Induction at the beginning with galactose 1%. Plus extra shot at 9 hours. | *Induction at the beginning with galactose 1%. Plus extra shot at 9 hours. | ||
*Left during two days. | *Left during two days. | ||
| - | |- | + | |} |
| - | | <center>''' | + | For the first time, mutant strains 76Δ and 175Δ significantly heated their medium up. Important increases in temperature were observed at the end of the experiment. However, temperature peaks after 40-50 hours might not be associated with thermogenin, since oxygen presence, which is imperative for aerobic respiration, might be virtually absent after long incubation times into a sealed LCC. In order to have an oxygen-rich environment allowing thermogenin to be active, higher initial O.D. and a vigorous shaking were introduced in the following experiments. |
| - | + | <br> | |
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | {| | ||
| + | | <center><font face="trebuchet ms" style="color:#047DB5" size="4">'''September 24th'''</font></center> | ||
| + | [[Image:V_done24_9.jpg|700px]]<br> | ||
| | | | ||
*Volume: 100 ml | *Volume: 100 ml | ||
| Line 72: | Line 67: | ||
*Induction at the beginning with galactose 1%. | *Induction at the beginning with galactose 1%. | ||
|} | |} | ||
| + | The new conditions (275 rpm shaking and initial O.D. 0.6) were used in this experiment. Mutant strains 76Δ and, particulary, 175Δ , heated up compared to UCP-. UCP+ also produced a significant increase in temperature after 5 to 17 hours. | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | {| | ||
| + | |<center><font face="trebuchet ms" style="color:#047DB5" size="4">'''August 29th'''</font></center> | ||
| + | [[Image:V_halfdebuti_29_8.jpg|700px]]<br> | ||
| + | | | ||
| + | *Volume: 100 ml | ||
| + | *SP medium | ||
| + | *275 rpm and slightly tilt. | ||
| + | *Initial OD: 0,6 | ||
| + | *Induction at the beginning with galactose 1%. | ||
| + | |} | ||
| + | Vigorous shaking (275 rpm) and a high cell concentration (O.D. 0.6) allowed a fast heating of 76Δ and 175Δ . Mutant strain 76Δ started heating its culture after only a couple of hours, whereas 175Δ heated the culture after between 7 and 15 hours. This difference in thermal activity is in concordance with the delayed growth of 175Δ compared to 76Δ (see [[Team:Valencia/Project/Lab_work/2_experiments#Growth Kinetics | Growth Kinetics]] below). This figure is representative (ten independent repetitions yielded similar results) of most of the experiments performed. | ||
| + | |||
| + | |||
| + | |||
| + | <h3> Growth Kinetics </h3> | ||
| + | Besides monitoring temperature evolution, we also compared the growth rate of the cultures. For this purpose, we measured O.D. of the cultures. O.D. was measured every ninety minutes for a nine hour period. We carried out this experiment under both normal culture conditions ( Erlenmeyer flasks shaken at 30ºC ) and with our thermally isolated LCCs. | ||
| + | |||
| + | This measurement was useful in order to determine the [[Team:Valencia/Project/Modeling#Black box model of temperature increase produced by thermogenin | growing parameters used in the modeling]] of our system. Culture growth characterization through O.D. indirectly confirmed the expression and functional activity of UCP1. As expected, mutant strains (Gly175Δ and Gly76Δ) exhibited a delayed growth kinetics indicating that uncoupling activity was present. UCP- and UCP+ grew faster.The former does not produce functional thermogenine whereas the later is not functional in absence of fatty acids. These results, indicating the expression of active thermogenine, confirm those on temperature increases we found with the LCC. | ||
| + | |||
| + | |||
| + | Strains growth equations: | ||
| + | {| | ||
| + | |[[Image:growthucp-.jpg|left|450px]] | ||
| + | |[[Image:growthucp++.jpg|right|450px]] | ||
| + | |- | ||
| + | |[[Image:growthucp76.jpg|left|450px]] | ||
| + | |[[Image:growthucp175.jpg|right|450px]] | ||
| + | |} | ||
| + | |||
| + | <h3>Conclusions </h3> | ||
| + | |||
| + | Mutant strains 175Δ and 76Δ significantly heat up their medium compared to UCP- and UCP+ (the latter yielding irreproducible results). The increase in temperature was obtained during the first highly aerobic phase of the culture and it is thus associated with the phase were thermogenin is active. Additionally, these results implying a high activity of thermogenin of the mutants are in concordance with the delayed growth rate compared to UCP+ and UCP-. In some experiments, a second peak was found for the mutant strains after very long incubation times. We hypothesized that this peak is related with ethanol-based respiration during the end of the culture (cultures smelt like champagne after such long incubations). The increase in temperature associated with the activity of thermogenin we found for 175Δ and 76Δ is the first report on measurable temperature increase in UCP-expressing yeasts. | ||
| + | |||
| + | |||
<h3> Troubleshooting </h3> | <h3> Troubleshooting </h3> | ||
| - | In order to establish the final conditions and | + | In order to establish the final conditions and protocols, we have gone through many different experiments. |
<ol>'''Low shaking speed, large volume and low initial O.D.''' <br> | <ol>'''Low shaking speed, large volume and low initial O.D.''' <br> | ||
| - | At the beginning | + | At the beginning these were the conditions for our LCC. However, since the induction should be done at higher cell densities, we had to wait three hours before induction. <br> |
*We did not obtain any encouraging results. | *We did not obtain any encouraging results. | ||
*Besides, we obtained weird oscillations in most of our experiments (as described in the [[Team:Valencia/Project/Lab_work#Troubleshooting|LCC troubleshooting]]). | *Besides, we obtained weird oscillations in most of our experiments (as described in the [[Team:Valencia/Project/Lab_work#Troubleshooting|LCC troubleshooting]]). | ||
| Line 83: | Line 119: | ||
| - | <ol>''' | + | <ol>'''Media variations''' <br> |
| - | As we were obtaining any results we desperately tried other | + | As we were not obtaining any results, we desperately tried other culture broths. |
| - | *[[Team:Valencia/Notebook/Protocols#YPKAc Medium | YPKAc Medium ]]: In this medium containing acetate, yeast is | + | *[[Team:Valencia/Notebook/Protocols#YPKAc Medium | YPKAc Medium ]]: In this medium containing acetate, yeast is supposed to only respire rather than ferment. We tried it just in case the problem was that electron transport chain was inhibited. |
| - | *Palmitate: although our Gly175Δ and Gly76Δ mutants do not need this compound, we tried and added it in case it made any difference for UCP+ strain. We tried and added it to both SP medium and YPKAc medium. | + | *Palmitate: although our Gly175Δ and Gly76Δ mutants do not need this compound for thermogenine activation, we tried and added it in case it made any difference for UCP+ strain. We tried and added it to both SP medium and YPKAc medium. |
*We did not obtain any encouraging results either. | *We did not obtain any encouraging results either. | ||
</ol> | </ol> | ||
| Line 92: | Line 128: | ||
<ol>'''Increased shaking speed and reduced volume'''<br> | <ol>'''Increased shaking speed and reduced volume'''<br> | ||
| - | In other to allow proper respiration the yeast culture in the LCCs, we tried | + | In other to allow proper respiration of the yeast culture in the LCCs, we tried to increase the speed of shaker. Additionally, we reduced the volume for the yeast culture to have more oxygen available. |
| - | *We obtained temperature | + | *We obtained temperature increases for the first time. |
| - | + | ||
*Besides, the weird oscillations observed before disappeared. | *Besides, the weird oscillations observed before disappeared. | ||
</ol> | </ol> | ||
| Line 100: | Line 135: | ||
<ol>'''Higher O.D.'''<br> | <ol>'''Higher O.D.'''<br> | ||
| - | Instead of starting the experiment at a lower O.D. and waiting several hours before inducing, we thought it would be better to start at a higher O.D. | + | Instead of starting the experiment at a lower O.D. and waiting several hours before inducing, we thought it would be better to start at a higher O.D. This way, we would be sure that the O.D. at the time of induction was the same for the four strains. |
| - | *In order to do | + | *In order to do so, we designed the two inoculum protocol described above. With this protocol, we intend to reach an high O.D. without having too much glucose. We used SP medium for the inoculum in order to avoid adding too much glucose (SD medium is glucose rich) to the LCCs. |
| - | *We obtained temperature | + | *We obtained temperature increases and we were able to reproduce it. |
| - | *We tried and | + | *We tried and began the experiment with an even higher O.D. but we have not obtained the encouraging results yet. |
</ol> | </ol> | ||
<ol>'''Initial temperature'''<br> | <ol>'''Initial temperature'''<br> | ||
| - | Even after obtaining results that | + | Even after obtaining results showing that mutants increased the temperature of the culture, we obtained weird results since none of the strains behaved as wished. We realized the disturbing factor of those experiments was the initial temperature of the culture, higher than that corresponding to the successful experiments. |
*We put a lot more effort in starting at the same temperature every time. | *We put a lot more effort in starting at the same temperature every time. | ||
*Nevertheless, this temperature inside the calorimeter depends on the room temperature. And room temperature can significantly change depending on the outside temperature. | *Nevertheless, this temperature inside the calorimeter depends on the room temperature. And room temperature can significantly change depending on the outside temperature. | ||
| Line 134: | Line 169: | ||
*Induction at 3 hours (approximate OD 0.15) with galactose 0.4% | *Induction at 3 hours (approximate OD 0.15) with galactose 0.4% | ||
|| | || | ||
| - | |||
*Weird oscillations | *Weird oscillations | ||
*Actual O.D. at the moment of induction different for the strains and different from predicted. | *Actual O.D. at the moment of induction different for the strains and different from predicted. | ||
| Line 166: | Line 200: | ||
*Induction after 5 hours with galactose 0.4%. Actual OD between 0,8 – 1,6 | *Induction after 5 hours with galactose 0.4%. Actual OD between 0,8 – 1,6 | ||
|| | || | ||
| - | * | + | *Inconclusive results. |
|-style="background:#FFF2D2" | |-style="background:#FFF2D2" | ||
| | | | ||
| Line 227: | Line 261: | ||
*Induction at the beginning with galactose 1%. | *Induction at the beginning with galactose 1%. | ||
|| | || | ||
| - | * | + | *O.D. at 2 is too high to begin with. |
| - | + | ||
|} | |} | ||
<div style="padding: 10px; width: 500px; color: #000000; background-color: #FFB428"> | <div style="padding: 10px; width: 500px; color: #000000; background-color: #FFB428"> | ||
| - | <center><font face="trebuchet ms" style="color:#047DB5" size="3"> [[Team:Valencia/Project/Lab_work | <font color="#047DB5">'''LCC Construction'''</font>]] << '''Hot Yeast Experiments ''' >> [[Team:Valencia/Project/Modeling | <font color="#047DB5">'''Modeling'''</font>]]</font> | + | <center><font face="trebuchet ms" style="color:#047DB5" size="3"> [[Team:Valencia/Project/Lab_work | <font color="#047DB5">'''1.LCC Construction'''</font>]] << '''2.Hot Yeast Experiments ''' >> [[Team:Valencia/Project/Modeling | <font color="#047DB5">'''3.Modeling'''</font>]]</font> |
</center> | </center> | ||
</div style> | </div style> | ||
Latest revision as of 00:44, 30 October 2008
2.-Demonstration that thermogenin-expressing yeast strains can heat their own broth medium.
Materials
In our experiments we worked with the the following Saccharomyces cerevisiae strains kindly handed by [http://www.cbm.uam.es/mitolab/fichapersonal.aspx?idpersona=6 Eduardo Rial] :
- UCP+ : UCP1 gene with Gal7 promoter.
- UCP- : UCP1 antisense gene with Gal7 promoter. It is used as control strain.
- Gly175Δ : UCP1 mutant sequence, Glycine 175 has been deleted.
- Gly76Δ : UCP1 mutant sequence, Glycine 76 has been deleted. These two strains have increased uncoupling activity and do not need fatty acids to be functional.
We used plasmid pYeDP as our vector in Saccharomyces cerevisiae.
Method of culturing UCP1-producing yeast strains for heat production
Each of our experiments entails the following steps:
- We prepare a 30ml inoculum for each strains in 100ml Erlenmeyer flasks with SD medium. We leave it overnight in the 28ºC stove.
- We prepare a second inoculum for each strain, this time 100ml SP medium in 1L Erlenmeyer flasks. The initial O.D. of this second inoculum is adjusted to 0.2. We leave it several hours in the 28ºC stove.
The objective of this two-step protocol is to be able to start the experiment with a higher O.D. in a medium with little or no glucose. In summary: first, we grow our strains in a liquid medium with glucose (SD medium), in which they have a better growth rate. Second, we use SP medium because it has a really small amount of glucose.
- We start the experiment in the LCCs with a volume of 100ml of SP medium, adjusted to O.D. of 0.6. We heat the medium up to aproximately 28ºC inside the LCC.
- We add 1% galactose to each of the LCCs containing the four yeast strains. This sugar is the UCP1 gene Gal7 promoter inductor and it is therefore needed for the production of UCP1.
- We monitor the temperature evolution in the LCCs thanks to our LCC system.
Experiment results
|
For the first time, mutant strains 76Δ and 175Δ significantly heated their medium up. Important increases in temperature were observed at the end of the experiment. However, temperature peaks after 40-50 hours might not be associated with thermogenin, since oxygen presence, which is imperative for aerobic respiration, might be virtually absent after long incubation times into a sealed LCC. In order to have an oxygen-rich environment allowing thermogenin to be active, higher initial O.D. and a vigorous shaking were introduced in the following experiments.
| |
|
The new conditions (275 rpm shaking and initial O.D. 0.6) were used in this experiment. Mutant strains 76Δ and, particulary, 175Δ , heated up compared to UCP-. UCP+ also produced a significant increase in temperature after 5 to 17 hours.
|
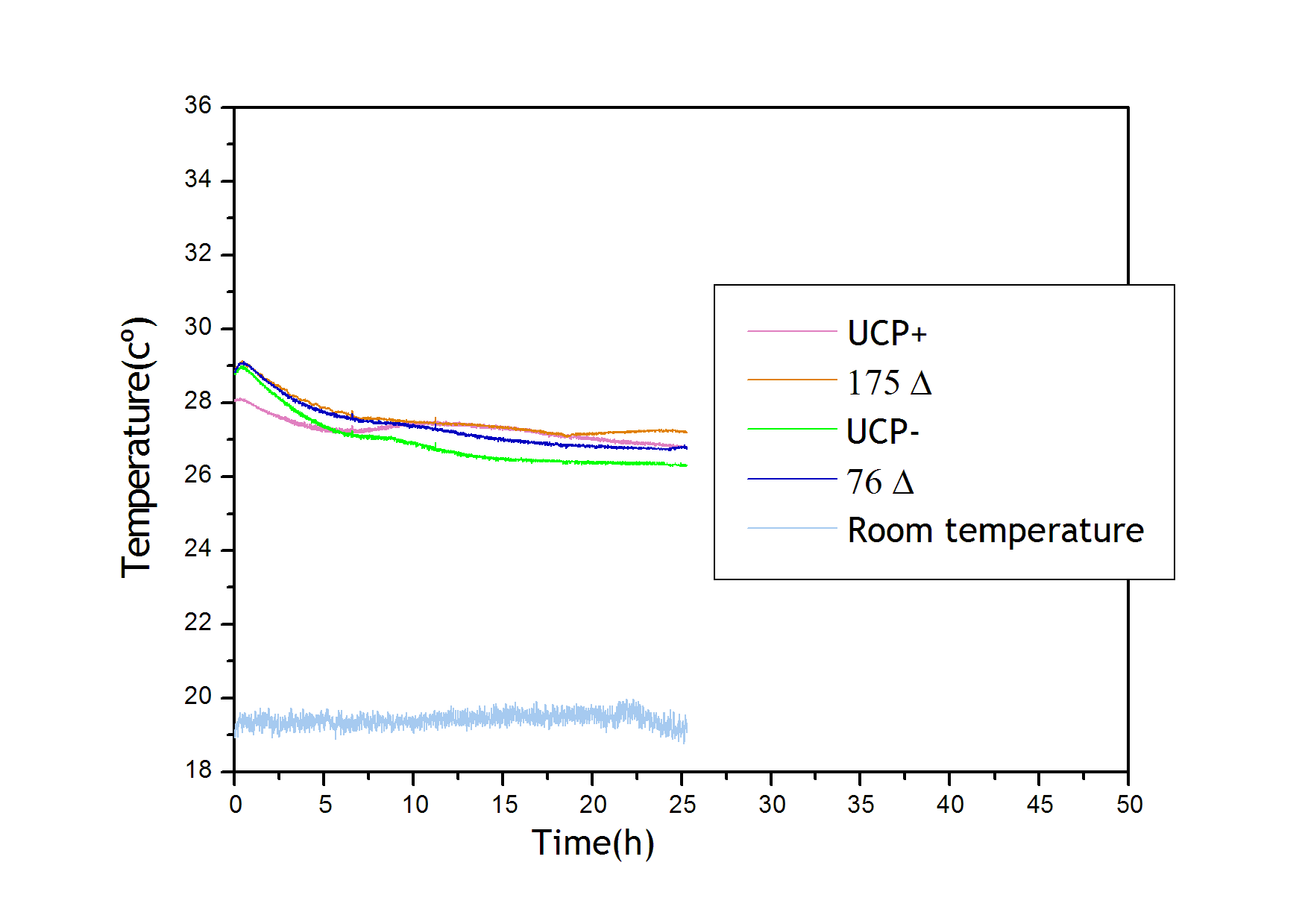
Vigorous shaking (275 rpm) and a high cell concentration (O.D. 0.6) allowed a fast heating of 76Δ and 175Δ . Mutant strain 76Δ started heating its culture after only a couple of hours, whereas 175Δ heated the culture after between 7 and 15 hours. This difference in thermal activity is in concordance with the delayed growth of 175Δ compared to 76Δ (see Growth Kinetics below). This figure is representative (ten independent repetitions yielded similar results) of most of the experiments performed.
Growth Kinetics
Besides monitoring temperature evolution, we also compared the growth rate of the cultures. For this purpose, we measured O.D. of the cultures. O.D. was measured every ninety minutes for a nine hour period. We carried out this experiment under both normal culture conditions ( Erlenmeyer flasks shaken at 30ºC ) and with our thermally isolated LCCs.
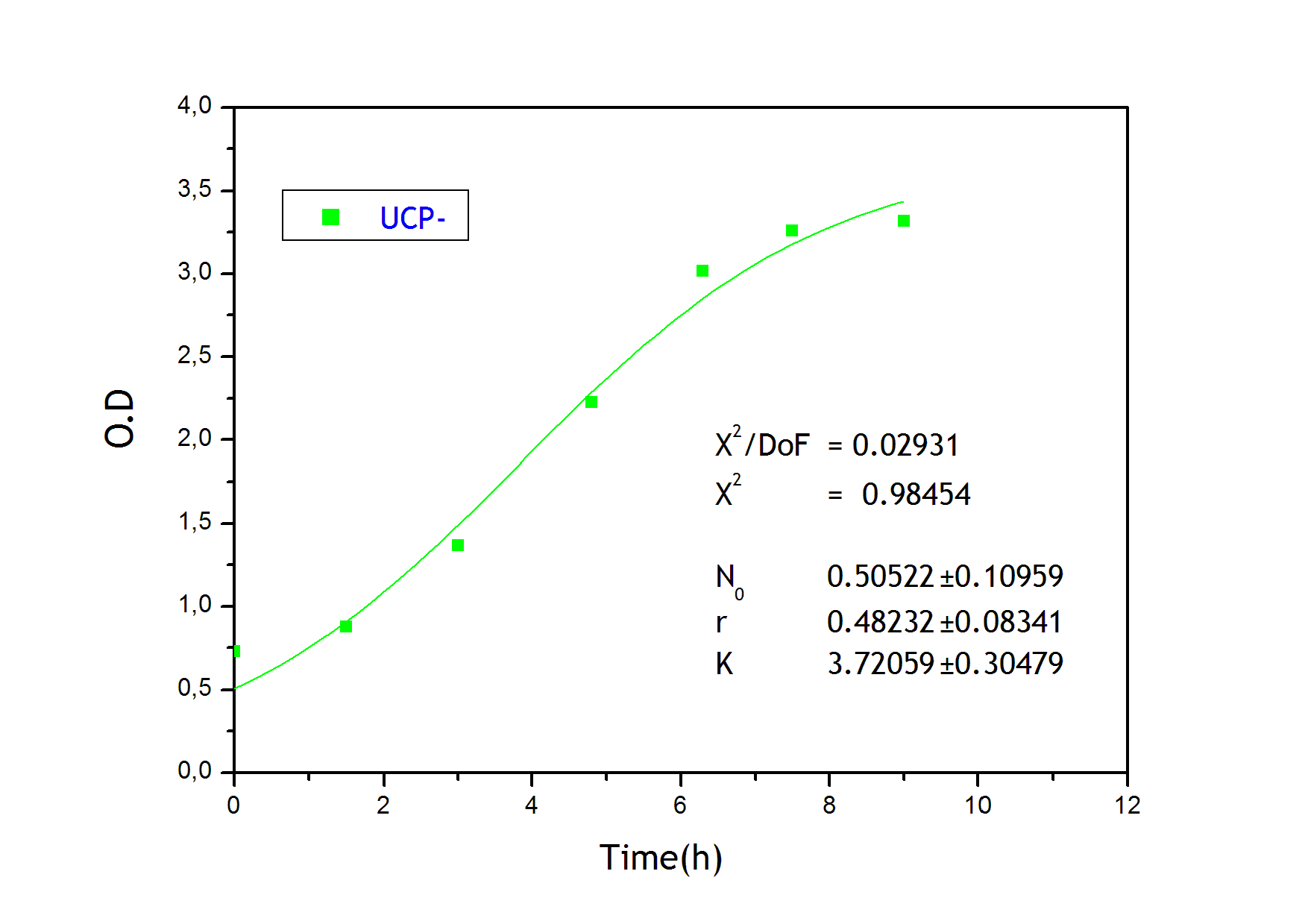
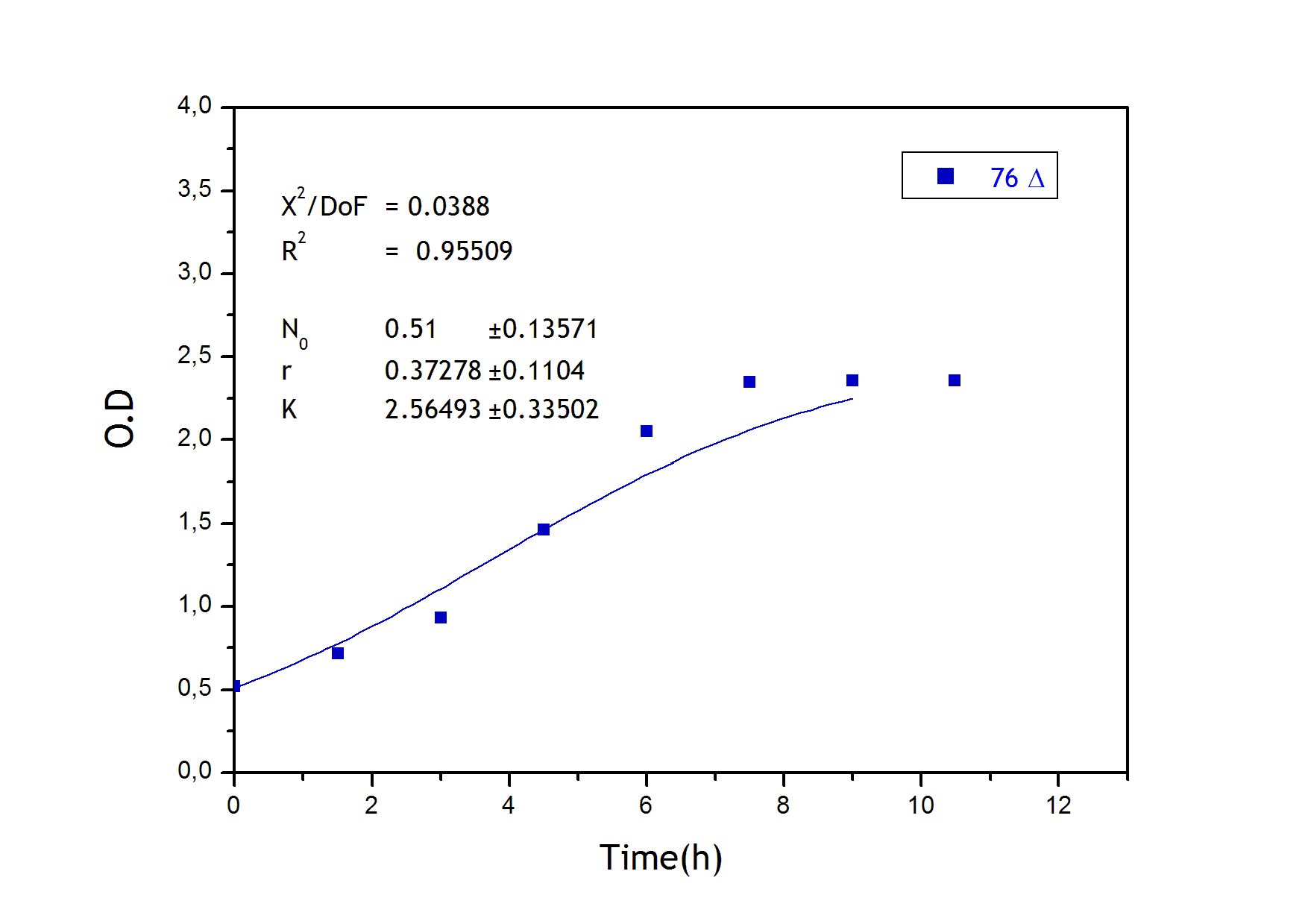
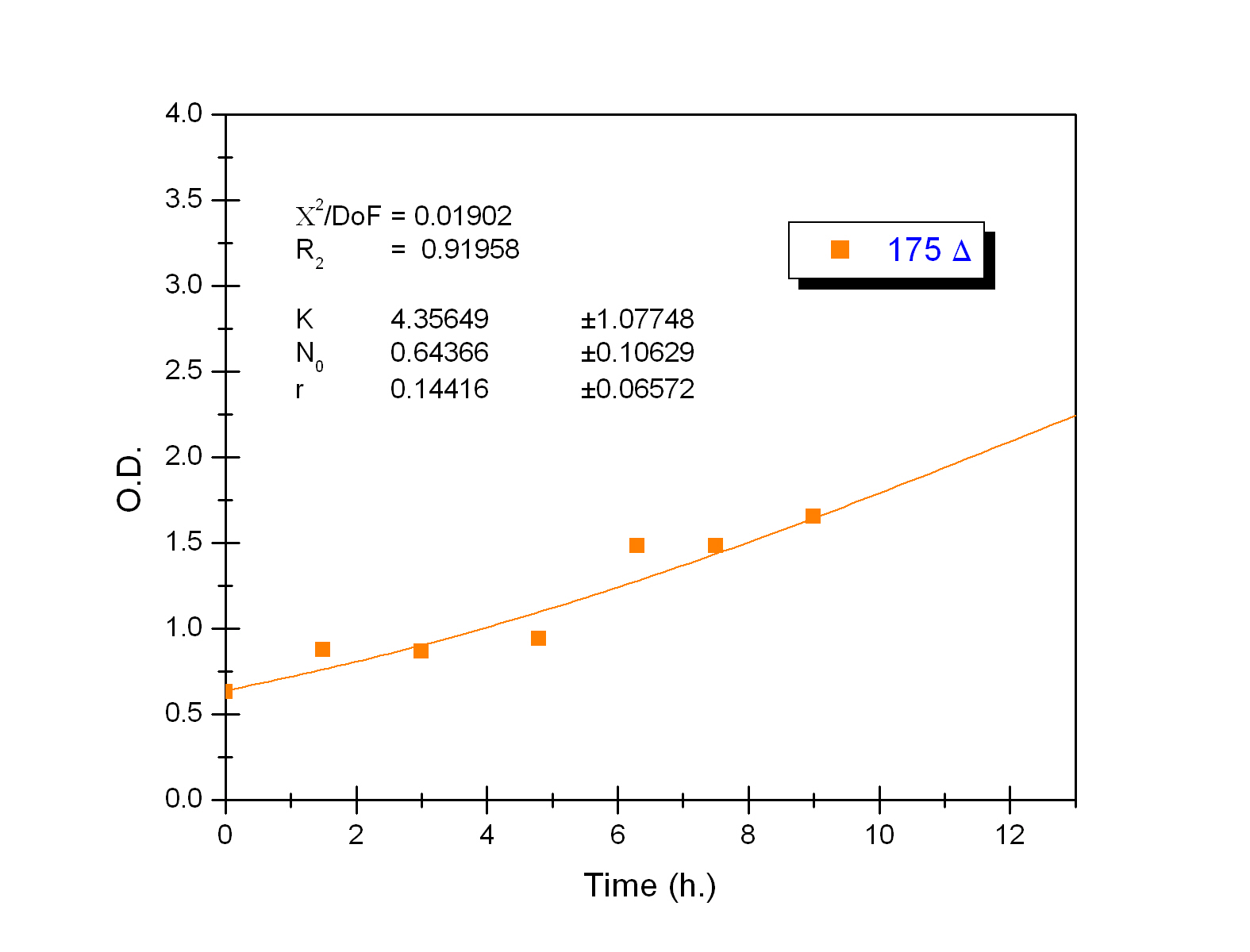
This measurement was useful in order to determine the growing parameters used in the modeling of our system. Culture growth characterization through O.D. indirectly confirmed the expression and functional activity of UCP1. As expected, mutant strains (Gly175Δ and Gly76Δ) exhibited a delayed growth kinetics indicating that uncoupling activity was present. UCP- and UCP+ grew faster.The former does not produce functional thermogenine whereas the later is not functional in absence of fatty acids. These results, indicating the expression of active thermogenine, confirm those on temperature increases we found with the LCC.
Strains growth equations:
Conclusions
Mutant strains 175Δ and 76Δ significantly heat up their medium compared to UCP- and UCP+ (the latter yielding irreproducible results). The increase in temperature was obtained during the first highly aerobic phase of the culture and it is thus associated with the phase were thermogenin is active. Additionally, these results implying a high activity of thermogenin of the mutants are in concordance with the delayed growth rate compared to UCP+ and UCP-. In some experiments, a second peak was found for the mutant strains after very long incubation times. We hypothesized that this peak is related with ethanol-based respiration during the end of the culture (cultures smelt like champagne after such long incubations). The increase in temperature associated with the activity of thermogenin we found for 175Δ and 76Δ is the first report on measurable temperature increase in UCP-expressing yeasts.
Troubleshooting
In order to establish the final conditions and protocols, we have gone through many different experiments.
- Low shaking speed, large volume and low initial O.D.
- We did not obtain any encouraging results.
- Besides, we obtained weird oscillations in most of our experiments (as described in the LCC troubleshooting).
At the beginning these were the conditions for our LCC. However, since the induction should be done at higher cell densities, we had to wait three hours before induction.
- Media variations
- YPKAc Medium : In this medium containing acetate, yeast is supposed to only respire rather than ferment. We tried it just in case the problem was that electron transport chain was inhibited.
- Palmitate: although our Gly175Δ and Gly76Δ mutants do not need this compound for thermogenine activation, we tried and added it in case it made any difference for UCP+ strain. We tried and added it to both SP medium and YPKAc medium.
- We did not obtain any encouraging results either.
As we were not obtaining any results, we desperately tried other culture broths.
- Increased shaking speed and reduced volume
- We obtained temperature increases for the first time.
- Besides, the weird oscillations observed before disappeared.
In other to allow proper respiration of the yeast culture in the LCCs, we tried to increase the speed of shaker. Additionally, we reduced the volume for the yeast culture to have more oxygen available.
- Higher O.D.
- In order to do so, we designed the two inoculum protocol described above. With this protocol, we intend to reach an high O.D. without having too much glucose. We used SP medium for the inoculum in order to avoid adding too much glucose (SD medium is glucose rich) to the LCCs.
- We obtained temperature increases and we were able to reproduce it.
- We tried and began the experiment with an even higher O.D. but we have not obtained the encouraging results yet.
Instead of starting the experiment at a lower O.D. and waiting several hours before inducing, we thought it would be better to start at a higher O.D. This way, we would be sure that the O.D. at the time of induction was the same for the four strains.
- Initial temperature
- We put a lot more effort in starting at the same temperature every time.
- Nevertheless, this temperature inside the calorimeter depends on the room temperature. And room temperature can significantly change depending on the outside temperature.
Even after obtaining results showing that mutants increased the temperature of the culture, we obtained weird results since none of the strains behaved as wished. We realized the disturbing factor of those experiments was the initial temperature of the culture, higher than that corresponding to the successful experiments.
Here you can see a summary of our experiments and the different conditions for them.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 "
"