Team:KULeuven/Brainstorm
From 2008.igem.org
m |
m |
||
| Line 1: | Line 1: | ||
{{:Team:KULeuven/Tools/Styling}} | {{:Team:KULeuven/Tools/Styling}} | ||
| + | {{:Team:KULeuven/Tools/Scripting}} | ||
{{:Team:KULeuven/Tools/Header}} | {{:Team:KULeuven/Tools/Header}} | ||
Revision as of 14:52, 3 October 2008
Favourite previous iGEM projects
Nathalie Busschaert
- [http://parts.mit.edu/igem07/index.php/Imperial/Infector_Detector/Introduction Infector detector] - Imperial College 2007
- [http://parts.mit.edu/igem07/index.php/Edinburgh Self-flavouring yoghurt] - Edinburgh 2007
- [http://parts.mit.edu/igem07/index.php/Paris Synthetic Multicellular Bacterium] - Paris 2007
Jonas Demeulemeester
- [http://parts.mit.edu/igem07/index.php/Ljubljana Virotrap Ljubljana 2007]
- [http://parts.mit.edu/igem07/index.php/Princeton RNAi enhanced logic circuit Princeton 2007]
- Other nice parts/devices:
- Caltech: Riboswitch design for targeted cell death/molecular sensor
- Cambridge: Inducible bigger pore protein for E.coli
- Harvard: Quorum-sensing & targeting!
- Melbourne: Red/blue light responsive system through chimeric photoreceptors-kinases
- Peking U: λ-based bistable switch = very powerful
- UCSF: compartmentalization! Rewired MAPK cascade signaling through scaffolds ≅ circuit board
Jan Mertens
- [http://parts.mit.edu/igem07/index.php/Ljubljana Virotrap Ljubljana 2007]
- [http://parts.mit.edu/igem07/index.php/Berkeley_UC Bactoblood]
Benjamien Moeyaert
- [http://openwetware.org/wiki/IGEM:Harvard/2006/DNA_nanostructures Harvard 20006: nanostructured DNA containers]
- [http://parts.mit.edu/igem07/index.php/Berkeley_UC Bactoblood]
Stefanie Roberfroid
- [http://parts.mit.edu/igem07/index.php/Cambridge Bacteria Online]
- [http://parts.mit.edu/igem07/index.php/Berkeley_UC Bactoblood]
- [http://parts.mit.edu/igem07/index.php/Princeton RNAi enhanced logic circuit]
- some other nice ideas
- [http://parts.mit.edu/igem07/index.php/Edinburgh Self-flavouring yoghurt]
- Detection of metals: [http://parts.mit.edu/igem07/index.php/Brown Lead], [http://parts.mit.edu/igem07/index.php/Saint_Petersburg Copper]
Hanne Tytgat
- [http://parts.mit.edu/igem07/index.php/Berkeley_UC Bactoblood]
- [http://parts.mit.edu/igem07/index.php/MIT Sensing & removing Hg ions - MIT 2007]
- [http://parts.mit.edu/igem07/index.php/Imperial/Infector_Detector/Introduction Infector detector]
Elke Van Assche
- [http://parts.mit.edu/wiki/index.php/MIT_2006 Eau d'E.coli MIT 2006]
- [http://parts.mit.edu/igem07/index.php/Berkeley_UC Bactoblood Berkeley UC 2007]
- [http://parts.mit.edu/igem07/index.php/Princeton RNAi enhanced logic circuit Princeton 2007]
Nick Van Damme
- [http://parts.mit.edu/igem07/index.php/Davidson_Missouri_W Bacterial Computer]
--> idea: solve a nice mathematical problem
- several electronical/biological components to build an entire complex combinational logic system
- [http://parts.mit.edu/igem07/index.php/USTC Extensible Logic Circuit in Bacteria]: both components and linking
- [http://parts.mit.edu/igem07/index.php/Valencia Comparator]
- [http://parts.mit.edu/igem07/index.php/Bologna Schmitt trigger]
--> idea: build an integrator to solve your own ODE's, also build a differentiator to make a PID-controller
Antoine Vandermeersch
- [http://parts2.mit.edu/wiki/index.php/University_of_Texas_2006 Texas 2006: Edge Detector]
- [http://parts.mit.edu/igem07/index.php/Rice/Project_B:_Quorumtaxis Rice 2007: Quorumtaxis]
- [http://parts.mit.edu/igem07/index.php/Berkeley_LBL Berkeley LBL 2007: Solar Bacter]
Sigrid De Keersmaecker
- [http://parts.mit.edu/igem07/index.php/MIT Sensing & removing Hg ions - MIT 2007]
- [http://parts.mit.edu/igem07/index.php/Edinburgh Self-flavouring yoghurt - Edinburgh 2007]
- [http://parts.mit.edu/igem07/index.php/Missouri_Miners Biological Timer - Missouri Miners 2007]
- [http://parts.mit.edu/igem07/index.php/Ljubljana Virotrap - Ljubljana 2007]
- [http://parts.mit.edu/igem07/index.php/Taipei/Taipei GlucOperon - Taipei 2007]
- [http://parts.mit.edu/igem07/index.php/Berkeley_LBL Solar Bacter - Berkeley_LBL 2007]
- [http://parts.mit.edu/igem07/index.php/Berkeley_UC Bactoblood - Berkeley_UC 2007]
iGEM judging tracks
Check out the iGEM judging page!
iGEM idea exchange
[http://openwetware.org/wiki/IGEM:Idea_exchange Idea exchange - iGEM ideas posted by other teams]
General considerations for our project
We had a little chat in the lab (Sigrid, Jos, Inge and myself) about a good project for iGEM. We have been exploring the idea of synthetic biology for some time now, and I guess we should consider some practical issues/ideas when choosing our project:
- It is not easy to manipulate whatever bacterium. We should best stick to the modelorganisms we are currently studying, for example E. coli, Salmonella, Rhizobium, Pseudomonas... Working with gram positives is much less trivial and basic expertise is available for Lactobillus but basic genetic tools (transformation,…) available for E. coli are not right away applicable to other organisms.
- I attended a Systems biology meeting ESF 2008 where Ron Weiss explained how he used a bacterial to manipulate eukaryotic cells. A very cool application but his lab worked on it for more than 5 years before they had any results. We should not underestimate the difficulty of these “biomedical” projects.
- Also, all applications where a bacterial cell will be used within a eukaryotic organism (medical applications) are far from being ethically approved. So realize we can not yet test these applications.
To find a project that is doable in a few months, I think we should take into account these issues:
- Work on an organism which is easy to manipulate, preferentially E. coli so that we can recycle already existing and characterized Biobricks.
- Clearly define a few modules which need to be simulated, constructed and tested.
- If we design a novel bacterium with a medical application, focus on an application for external use (for instance treating herpes labialis?). It circumvents many ethical problems while still providing sufficient proof of concept for the future.
- Modules could be devised as such that they are quite simple but general such that they can fit in the many different biological/medical applications we can think of later on.
- We were thinking of for instance a bacterial timer. Such timer could serve as a suicidal system where the bacterium as long as it senses a certain “molecule” remains active but as soon as the molecule disappears the timer is switched on and if no molecule appears within a certain time interval the bacterium commits suicide. This would be a smart way of providing “clearance” in a potential application. The “molecule” could for instance be a cancer cell or a viral protein. This would also nicely fit the idea 3, as a module to include.
- Another useful module would be a bacterial system that senses a eukaryotic protein and transduces the sensed signal into a certain behavior. As mentioned in idea 3, and remarked by Jonas, sensing the IL will be very challenging. Again, this module can be included in the general idea 3. We have been checking the literature and could not trace yet the existence of such bacterial sensing systems that could be modified for sensing eukaryotic proteins, although some possible leads exist. We believe two component systems with a sensor in the periplasm could be useful but more thorough literature checking should be done. Also here we have to define an easily tracable eukaryotic protein that can be made compatible with the eukaryotic sensor.
- We need to simulate the desired behavior of our module, determine the kinetic patterns of the different signal transduction systems, generate libraries of signal transduction components with different kinetic parameters from which we can choose. => this is for every module quite some work. So we can not build too many different modules and we have to plan how to parallelize this process and construct subparts in a general way. Maybe this way we can divide the team in some smaller subteams that can each have their own “subproject”.
- To test the functionality of the tools we could use dynamic FACS experiments with gfp.
Kathleen
Loose ideas
To have a space for wild ideas...
- Bacteria can sense, move, react to light, make light...but can they make a sound? Sound are waves, so could we do something with flagella? Or can we have them perform a hearable chemical explosion? Or have them react to sound? --Ingethijs 14:28, 29 May 2008 (UTC)
- About the physical process: a sound wave is a representation of local matter densities throughout space (varying in time), see it as a sphere (cell in the middle) growing bigger and all points on the sphere have the same density ideally. So produce this periodic density function, the cell would need to contract, relax, contract, relax, and so forth... or the flagella could move (might be rather inefficiënt though).
- The math: a sound wave is represented by 2 parameters: frequency and power(or intensity, which is the power per unit area). Power is the energy per time and will be related to loudness (decibel!)
- Frequency: in order to be able to stimulate the ear bones (don't know a better name), the frequency would have to lie in between 16-16000Hz (let's take 16-3400Hz, the frequency band used in telephony, containing the most informational frequencies). I assume a cell would have no problem with frequency.
- Loudness: the loundess in decibel should range from 30-100dB to produce a hearable sound, keep in mind that the loudness scale is logarithmic related to the intensity emitted by the cell. The intensity is the emitted power per unit area (so divide the power by the area of a sphere, and you'll notice that intensity lowers as the radius of the sphere increases, reason why distant sound are hard to hear). Given this, I doubt whether a cell, or even a colony of cells, would produce a hearable sound (in case of a colony they would need to contract and relax synchronous for constructive interference of the individual sound waves).
- So I think it's definitely possible, just not detectable with the human ear, no reason why sounds are produced by macroscopic muscles pushing air against membranes (the power difference). --Antoine
- Now I think of it, if the "organism" would be multicellular and spherical (there will be more options) it might become interesting if it were possible to "ignite" a signal somewhere in the middle, this way the middle cell(s) could transport a signal toward the outer regions and expand/contract synhronous alongside this signal. If this might work, the system could be modelled as a (continuous?) cascade of amplifiers and perhaps the right gain could be attained to produce a hearable sound.
- Other ideas mentioned in the brainstorm session:
- exploit magnetic properties - bacterial compass
- case of ferromagnetism, but because the atomic and molecular structure is so different, I wouldn't know where to start =S --Antoine
- genetic algorithm (optimization) - building blocks with standard recombination sites
- bacteria that can sequence (FRET?)
- exploit magnetic properties - bacterial compass
A first idea: cancer treatment with genetically modified blood cells
As cancer cells need a lot of energy to replicate themselves, they should be well provided with blood. Therefore, blood cells could be the right choice for in situ treatment of cancer. First, we should immobilize these blood cells on the cancer cells. Subsequently, these blood cells should secrete specific agents that reduce the activity of the cancer cells. (These 2 steps may come in handy if we want to split up in 2 subgroups) -Liesbeth
Notes
- Sounds like a great idea! [http://en.wikipedia.org/wiki/Avastin Anti-angiogenic therapy] is one of the big hopes for anti-tumor treatments. But let's keep in mind that angiogenesis (the formation of blood vessels) is only a late hallmark of tumors [http://www.google.be/url?sa=t&ct=res&cd=1&url=http%3A%2F%2Fwww.weizmann.ac.il%2Fhome%2Ffedomany%2FBioinfo05%2Flecture6_Hanahan.pdf&ei=-7E2SLbLGJCE1wblseHQDQ&usg=AFQjCNHirXaVMmdQNGl-72bk5jRva4106Q&sig2=L18rRG56VaP9wIB_mAENpA (more about these hallmarks of cancer -PDF)]. It is however a significant barrier to break through if the tumor has to grow past a certain (very limited) size. So this would be more like a therapy for later-stage malignancies, which would also be great because it's often the metastasis (the spreading of) of the tumor that is causing the more visible effects of the cancer. (pain, deterioration, ... and eventually, if untreated death). - Jonas 12:09, 23 May 2008 (UTC)
- Anyhow, if we proceed with this idea, it will be a challenge to get everything ready and produced in the erythrocyte (red blood cell) before it loses it's nucleus and thus also the ability to initiate de novo transcription. And to keep all this machinery silent in non-docked erythrocytes. I'm liking this challenge though. Besides this has an upside as well as I feel that consequences would be less severe in this non-cell if things go awry in the system. :) - Jonas 17:30, 23 May 2008 (UTC)
- I just thought of something that might be quite critical. If I recall correctly, there are 3 main ways in which tumors acquire blood supply.
- The first one is through a recapitulation of embryonic development. This is the recruitment of vascular endothelial precursors or the activation of local endothelium via factors like VEGF (angiogenic sprouting or intussusceptive growth). In this case, the 'vessels' of the tumor blood supply are lined mostly with endothelial cells which are actually NOT malignant, but are kind of working together with the tumor cells.
- A successful cancer metastasis (a secondary tumor, derived from the original) will co-opt blood vessels and these will thus also be lined mostly with endothelium cells.
- The third way to achieve blood supply is through vasculogenic mimicry, where the tumor cells actually DO line the bloodstream and mimic the normal vascular endothelium. Here tumor biomarkers should be directly displayed to the passing erythrocytes and would thus be potential targets for use in this approach.
- OK, now for my point. In all these cases the vessels are highly abnormal, both structurally and functionally. They've got many holes, inhomogeneous bloodflow, are leaky, ... so it's very likely there will be exposed markers we can focus on but this will probably not always be the case. But anyhow, I'm really liking this idea! - Jonas 14:17, 23 May 2008 (UTC)
- There will be a lot of challenges. Some things that popped in my head: 1)What is the progenitorcell we're going to work with. When you use stemcells, you have to differentiate them in vitro into red blood cells which is pretty delicate matter. 2)finding the right markers for the recognition that are not expressed on normal cells. 3) developing an assay that proves that it works. Whith tis idea there are a lot of factors that have to be taken in account and the question is if we will get a positive result in 3 months time. There's a lot of ongoing research and a lot of things ar not yet known. But I also like the idea, next year I'm going to do my thesis about lymphangiogenesis so I'm very interested in the subject :)- Jan
- On the biomarker issue. There's a lot of this kind of proteomics research going on at the Biology faculty/GHB. I hope we could pick up some molecules there? Because starting our own search for these and getting results in 3 months time would be quite hopeless indeed - Jonas 23:46, 23 May 2008 (UTC)
- I agree that working with RBC will be quite a challenge. But maybe we can use bacteria with the right proteins, just to proof the principle. If it works, another team (next year?) or research group can then try to apply this principle on RBC. Anyhow, I also really like this project. --BNathalie 16:18, 24 May 2008 (UTC)
- We could overcome the differentiation problem if we work with a mouse model for example. We harvest (or get them from someone) CD34+ hematopoietic progenitor cells (or other progenitors, I don't know), transfect these with our constructs + maybe an extra marker like green fluorescent protein (GFP) and then reintroduce these cells into the mouse. Differentiation should proceed normally then and we could harvest the modified and differentiated red blood cells (RBCs) by FACS analysis or something similar. The problem is that I've got no idea how long a procedure like this could take and we should be sure that our constructs work properly before trying this. We could also attempt myeloablative treatment on the mouse before reintroducing the transfected cells, this would make reharvesting a lot easier but once again, no idea how long this takes ... On the other hand, if we could harvest the modified RBCs, an in vitro assay should be quite feasible. - Jonas 22:06, 24 May 2008 (UTC)
- I've been thinking about the agent we have to deliver in order to kill the cancerous cells. We could deliver Reactive Oxygen Species (ROS) like hydroxyl radicals, superoxide, hydrogen peroxide or singlet oxygen to the tumor. This could potentially be very hazardous or lethal to the tumor cells since they often have defective DNA repair machinery and would thus be unable to cope with this amount of damaging. The erythrocyte vessel we target to the tumors is extremely well fitted to do this since it's packed with iron that could potentially catalyse the production of a lot of these ROS via the [http://www.lenntech.com/Fenton-reaction.htm Fenton reaction] etc. So all that should happen after the erythrocyte docks to the cancerous cells would be to destroy some hemoglobin, set some iron free in the red blood cell and let ROS be created locally! - Jonas 09:00, 24 May 2008 (UTC)
- One last thing. The entire erythrocyte metabolism has been [http://www.tbiomed.com/content/2/1/18 simulated] in silico (including links to hemoglobin). So this project could also provide enough entertainment for the more modeling-oriented amongst us :) - Jonas 23:12, 25 May 2008 (UTC)
A second idea: bacteria clean virusses in animals
This is an improvement of the idea of Ljubljana: we cannot reprogram the immune system, but we can reprogram bacteria. So, what we could do is make bacteria produce viral receptors (challenge 1) which are modified so that when a virus attaches to them, a restriction enzyme is transcribed (challenge 2). This RE degrades the viral DNA and the bacterial DNA, thus killing the bacterium. This way, the bacteria clean all virusses from the body. When this is established, we can induce a suicide signal for the bacteria (challenge 3). Big problems:
- Is it possible to make a eukaryotic virus attack a prokaryote (also a fundamental question)?
- Immunogenicity bacterium (cf. Bactoblood)! Bmoeyaert
- I'm probably risking to get banned for nagging because of this. :P I think the tricky part would be the preferential docking of the viruses on the bacteria if you've got about 10^14 of your own cells in your body. Even if only a small subset of these would be targets for the virus, I believe the ratio of bacteria to host cells would be quite low unless you allow a very large 'bacterial infection'. This problem might be overcome if the viral receptor density is way higher on the bacteria. - Jonas 17:52, 23 May 2008 (UTC)
- Cool stuff though, the opposite system exists. A bacterial infection could be treated by administering the specific bacteriophages, here the system can also co-evolve. Problem is that you have to know the bacterial strain of the infection in order to administer the correct phage, and this can take a while. - Jonas 17:52, 23 May 2008 (UTC)
- It's an appealing concept. An eukarotic virus only has to cross the celmembrane to get its content into the human cell. When we would use E.coli, the virus would have to get first through the outermembrane, then through the peptidoglycane and then through the innermembrane. So how does it get it in the cytoplasm? But maybe this is not necessary and is it sufficient to let the virus cross the outermembrane and come in the periplasm. If this induces the cell to do apoptosis en kill itself, including the virus, we've got a nice system. But like Jonas said, there will always be virusparticles that infect human cells, wich are in the majority. But still, it would be quite an accomplishment to get an eukariotic virus to infect a bacterium.-Jan
A third idea: Bacteria that control the amount of drugs
Generic goal
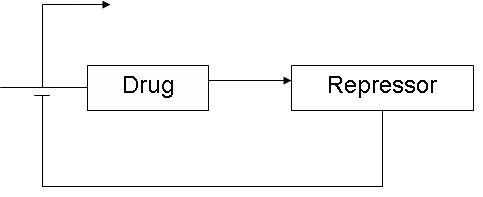
It's known that the effect of drugs on a human being is very dependent on the metabolism of that person. There are extensive metabolizers, people who have a normal response to a certain drug. Intermediate metabolizers show a low response and in poor metabolizers there's very little effect. Poor metabolizers need a higher amount of drugs to respond, where ultra fast metabolizers need a lower dose. These differences in the drugmetabolism in different people is a big concern, because a wrong dose can have severe consequences. There's a need for patient-focussed therapy. If we want to know which type of metabolizer a person is, we have to know his genotype. This is a very expensive and time-consuming procedure. Wouldn't it be nice to develop a two bacteria-system that can replace genotyping? I' m thinking of two bacteria that live together in the intestine. The first bacteria has a sensor function. It has to monitor if the amount of the drug that is present in the intestine, is efficient. This bacteria has to signal his observations to the second bacteria. This second bacteria is the drug-producer. Like some probiotics, that are already on the market, it produces the drug at the location where it's needed. The amount of drug it produces would be controlled by the other bacteria. In case the amount of treatment is enough, the first sensor-bacteria represses the second one. If the drug level drops again, the sensor-bacteria should re-activate the drug-producing one. In that way there's a feedback loop between the two bacterias and one can control the amount of treatment that's needed. --Hanne 19:27, 23 May 2008 (UTC)
- I don't think you necissarily need a 2 bacterial strain system and can also do it with one bacterial strain that has sensor- and drugproducer function. When it monitors the amount of drugs it can autoinduce itself to produce the drug and repress itself when it's enough. like the idea and think it's possible to do this in 3 months. It's also an idea in which I see our different skills can be integrated (the engineers can do modelling of pharmacokinetics for example), In the first 2 ideas that's maybe more difficult -Jan
- I also like this idea. Like Jan says it's a project were we are going to need all our different skills. Maybe this is more interesting for the engineers among us because it needs a lot of modeling. --stefanie
Application to Crohn's disease
- I would streamline the idea further:
- Use a single probiotic bacteria (lots of available expertise on probiotics in the team)
- Use a peptide or protein drug - probably a lot easier than synthetisizing a small molecule
- As probiotic bacteria live in the gut, peptide or protein drug could probably enter the blood stream
- Make a sensor for a signal that indicates the reaction to the treatment
- I was thinking about Crohn's disease (because it happens in the gut). Produce a peptide such as vasoactive intestinal peptide (potential treatment for Crohn). Sense the inflammatory response present in the gut. If high inflammation, increase drug production - up to some maximum dosage to avoid overdosing. Do not respond too fast to avoid overdosing.
- Crohn's expertise available in Leuven (P. Rutgeerts, S. Vermeiren) --Yves
- I also like this idea. However, you can't really control how long the bacteria stay inside your gut. So I think this is a system that is best used for chronic conditions (like Crohn's disease) that require continuous treatment. I like the example of Crohn's disease, because you can build in a good control devise to avoid overdosing. In this system, we need a bacteria that produces a drug only when: a) there is a low concentration of the drug inside the gut, and b) when the gut is inflamed. Practically, this means a receptor that will stop the production of the drug upon binding to the drug. I don't know how to sense inflammation though. --BNathalie 16:35, 24 May 2008 (UTC)
- I was indeed thinking of applications for chronic disease. --Y
- In the case of Crohn's, I think it would be quite doable to sense inflammation as there are many molecules typical of this process. We would have to look up which ones though. --Y
- An additional advantage is that Crohn's disease is characterized by local inflammation, so that the system would probably produce more drug exactly where it is needed most. --Yves
- Genetically modified Lactococcus lactis bacteria constitutively expressing Interleukin-10 (anti-inflammatory) are [http://www.ncbi.nlm.nih.gov/pubmed/16716759?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum already in stage I clinical trials] for the treatment of Crohn's disease. The dosage regulation by sensing could be a very nice addition to a system like this. I don't know however to what degree Crohn's disease is temporally variable and thus might benefit from such a system. - Jonas 23:28, 25 May 2008 (UTC)
- People in Leuven (Gastroenterology lab) are collaborating on the studies on IL-10-producing lactobacillus. This product is being developed by a spin-off of the Flanders Institute for Biotechnology (VIB) called Actogenix (www.actogenix.com) --Y
- Might be [http://www.nature.com/nature/journal/v453/n7195/full/nature07008.html interesting], only recently published. Benjamien
Control
- As an engineer this also seems the most challenging proposal. To regulate the proportion of drugs it seems that we are going to need a PID-controller (proportional with the amount already available, integrating to keep the level steady and differentiating to avoid responding too fast). It would be very challenging if we could also achieve this kind of controlling system in the bacteria. --Nick
- I think you see it somewhat wrong. It's about sensing what amount of drugs a certain patient needs. So there is a variable, not only in one patient, but also between different patients! It's about sensing how much every person needs. I think the idea of implementing this system for the treatment of Crohn's disease is really interesting. It's true that Lactobacillus lactis already delivers the drugs at the right spot in the body, but the control mechanism isn't there yet. So I think we could make an important improvement to this approach. This system can than serve as a model for treating other diseases. --Hanne 09:13, 26 May 2008 (UTC)
- The reference signal for the PID would simply be the local inflammation, but does this mean the bacteria would extract no information about the specific metabolism of the host (person)? If it would, the PID-controller could be optimised for every person by means of self-tuning (retrieving optimal parameters that characterise the PID-controller behaviour and determines it stability). Anyway, I like this idea a lot. EDIT: just saw Hanne's post, this is indeed what I ment --Avdmeers
- Ideally, the dosage would be patient specific and variable in space and time. In space, because the disease is not uniform across the intestine (some regions are in a better condition while others are worse). But I guess that because the mixing and diffusion that happens in a fluid environment, it is hard to control for production at a very specific spot on the intestine, except if the bacteria would stick to the intestine wall itself (might be possible with the right bacteria). However, patient specific dosage and time variability would be a very interesting advantage as not all patients have the same severity of the disease and as the disease progresses with up-and-downs ("The disease is characterized by active periods, known as flare-ups, followed by periods of remission, during which symptoms diminish or disappear altogether. Its cause is not known." - ehealthmd.com). Basically, the drug would be self-dosing. --Y
- The project does indeed bring together the interests of many people: a realistic medical application, work on bacteria that looks doable, and a tractable modeling problem based on well-known engineering principles (control engineering). --Y
- As a security mechanism, I guess that the bacteria are simply sensitive to antibiotics and thus a course of antibiotics is sufficient to stop the treatment altogether. --Y
- Possible extension for later: sensing adverse reaction. Some patients might respond badly to the treatment. We could have a system that senses physiological distress from the patient and then shuts down the drug production. This could be faster than giving antibiotics. Something like that might also work for patients who metabolize the drug differently (slower and faster) and for whom baseline level (i.e., for the same degree of inflammation) of the drug need to be lower or higher. --Yves
- By having a system with signaling molecule - nuclear receptor - response element - reporter/drug (such as PGE2-NR4A2 - NBRE - luciferase), we implement a proportional P controller. We can set the the constant K of the proportional controller by setting the strength of the promoter (for example, with multiple copies of the response element (3xNBRE in the colon cancer construct). --Y
- To get an integration I controller, we would need a construct that controls a protein that will accumulate in the bacteria and that then controls the reporter (signal - nuclear receptor - response element - intermediate transcription factor - accumulation - response element - reporter). --Y
- I have no idea about how to implement a differentiation D controller. --Y
- Depending on characteristics of the reference signal (local inflammation) and how it responds to the output signal (drugs concentration released) --- so the reference signal is in itself not independant of the output signal, there is an implicit coupling --- certain blocks could be omitted for simplification. The differentation term is ment for minimizing overshoots during rather abrupt transitions of the reference signal, so we could need information on time delays. --Antoine
- remark: mathematically speaking is the reference signal the setpoint of the system that must be reached, so i think it's better not to call the local inflammation the reference signal. The local inflammation is the output of our "proces" (a CV: controlled variable) which has to be compared to the reference signal (setpoint) that i assume you take equal to zero (we want to reach zero inflammation). the difference between these signals can then be considered as error-variable that is used as input to the controller. In that way the inflammation is indeed equal to the signal that is given to the controller ... --nick
- The [http://parts.mit.edu/igem07/index.php/Valencia/Controller#Sensibility_of_the_system_to_the_different_parameters| Valencia team] for example simplified their PID-controller to a regular P-controller. --Antoine
- That means we have found our challenge: first test their proportional controller (maybe try to improve it) and then add the integral (and maybe differential) controller. --nick
- for the integral/differential components, couldn't it be a good idea to implement the electronic equivalences and make somekind of 'simulink-diagram' that interconnects them?
- Found some nice [http://caltechbook.library.caltech.edu/226/| literature] of PID's including examples, it's in pdf, but chapters can be viewed seperately (PID's on chapter 10). Might be useful for review after exams, if the System Theory course lacks info, like parameter tuning. One of the authors occurs in the bibliography of feedback control in the System Theory course of Vandewalle, so I reckon it's worth it. --Antoine
- Maarten and I had a course on Computer Aided Control System Design (by prof. De Moor), so the background knowledge and (some) practice on (parameter) tuning for both proportional and integral controllers is already available. --nick
- I'm probably missing something here but I was wondering whether just one inflammation-sensing promotor regulating an output gene isn't all we need to have a PID-controller? In this simple sensor-promotor-output (eg IL-10) system your transcription will start when the sensor is activated. This is automatically proportional to your activation of the sensor. (so the P part, right?) The production of the mRNA/protein will always ramp up slowly untill a certain equilibrium is reached between export and synthesis. (the I part?) This equilibrium also automatically depends upon the sensor. Finally, if the input signal disappears, your output will automatically decrease slowly and not instantly, due to the mRNA still being present and able to make protein output. (D?) So my question again, isn't this sytem by itself a PID controller? (correct me if I'm wrong, because I'd never heard of a PID controller before this project) - Jonas 18:49, 2 June 2008 (UTC)
- what you're saying is that there's already some kind of easy built-in controlling system for controlling the output, but in that way you're not controlling the proces of [drug-administering to cure the inflammation]: there will be different time constants and ratios which aren't proportional to just the amount of inflammation. --nick
- I'm still not quite getting the difference, I can imagine you could change some time constants by adding parts but would that really make a difference in a biological site as 'chaotic' as an inflamed gut? Is the goal of the system more like a platform for feedback- and parameter-controlled biological drug delivery? - Jonas 08:04, 3 June 2008 (UTC)
- Sounds to me like your system would be an open loop system, so there is indeed a built-in control system, but the output is not used to compare to what the level should be (zero local inflammation). Your input signal remains only the sensor, meaning there would be no feedback (the system doesn't do a 'measurement' of its own output and calculate the error signal out of it). Closed loop system (PID) lead to better performance however, cause it would try to reduce that error signal as close to zero as possible (and can do this very effectively), as opposed to simply sense the local inflammation and release drugs. --Antoine
- Thought of something else though. To avoid overshooting the dosage level, you should also be aware of how many other bacteria are in the gut producing the drug, so the control should be sensing this as well. Should be feasible to insert this as well since there are parts available in the registry to do this through quorum sensing. - Jonas 08:04, 3 June 2008 (UTC)
- The PID would be a subsystem integrated in a logical circuit, making the decision whether to activate the PID by keeping in mind things like quorum-sensing, the psychological distress and so on. It's good it's brought up, definitely an issue that needs to be sorted out. --Antoine
- indeed a good point: normally a process is controlled by only one PID-controller, but now we have multiple controllers which have to co-operate to control the proces: distributed control. The problem is getting more and more interesting (and challenging)!!! nick
- no idea what you're talking about, but I like it! :) - Jonas 19:15, 3 June 2008 (UTC)
- The difference between a proportional and an integral controller would probably come from the speed at which the response transcript or protein is degraded. If the external signal triggers a gene whose transcript or protein is degraded fairly quickly, then the amount of protein present in the bacterial cell is PROPORTIONAL to the signal that drives this gene at this moment. If the response transcript or protein degrades slowly, then the amount of protein present in the bacterial cell is the amount that has been accumulated (= INTEGRAL) over a period of time. It is a bit unclear however how two such signals could be combined (i.e., added) into a final output signal. --Yves
Sensing
- You could sense several cytokines, such as TNF-alpha, interleukin-1, interleukin-6 as a measure of disease status. I guess for a cytokine, you could use a corresponding cytokine receptor as sensing device. --Y
- An important issue is whether those disease-marker proteins are measurable in the gut. I would assume so since they are probably produced at the inflammation site (but not sure about that). --Y
- Other important markers are fecal calprotectin (so most probably present in the gut) and C-reactive protein. --Y
- Like Yves, I was also thinking about what we could sense while remaining on the luminal side of the gut. The localisation of the cytokines might not be a problem since the surface of the gut lumen is severely damaged in the patient and even blood can be exposed. Since the inverse works; the IL-10 can get in, the others should be able to get out I presume. I don't know how long these proteins last in the gut though (because f degradation by other bacteria/our own proteases) and thus how long they are able to constitute a valid signal. But once again the Interleukin-10 in clinical trials seems to last long enough, so this might not be a problem either. - Jonas 16:09, 26 May 2008 (UTC)
- If we go through with this project, I feel like we should keep modularity of the system in mind. This could make the introduction of other sensors or other output molecules a lot easier if knowledge about the disease progresses. So we would basically be creating a platform for Crohn's or other inflammatory bowel diseases on which anyone could further build, switching inputs, outputs and stuff. And if we've got spare time during the summer we could implement extra sensors or switching output molecules ourselves. - Jonas 16:09, 26 May 2008 (UTC)
- PS: maybe Eicosanoids or other non-protein inflammatory molecules could also be used to detect the inflammation. These wouldn't be bothered by proteases and can travel through cell membranes so they should definitely be present in Liver Receptor Homologue-1the gut (if they are produced in Crohn's) and they can be easily picked up by nuclear hormone receptors. - Jonas 16:14, 26 May 2008 (UTC)
- Both measuring cytokines and eicosanoids seem viable options (see http://gut.bmj.com/cgi/content/full/46/4/487 with TNF-alpha and IL1-beta (and IFN-gamma) as cytokines, and Thromboxane B2 and prostaglandin E2 as eicosanoids). So it would be a question of what is easier for the experimental work. My impression though is that many eicosanoids are detected by transmembrane receptors rather than nuclear receptors. --Y
- We still have to face the problem of what organism to use and how it will sense what we want it to sense. For example L. lactis is gram-positive with a big layer of peptidoglycan shielding off it's membrane surface. So how will the signaling molecule be sensed? The TNF-alpa trimer is quite big. If we use a gram-negative like E. coli we will still have to have our signal transduced through the periplasm. This might prove to be quite tricky - Jonas 17:19, 26 May 2008 (UTC)
- Most eicosanoids do indeed bind to GPCR transmembrane receptors but some can also bind to PPAR subfamily nuclear receptors, these form heterodimers with the retinoic X receptors and can immediately induce gene expression. With a GPCR we'd have to construct a longer signal transduction cascade in the bug (which could allow additional regulation). Nuclear or membrane, it shouldn't make a big difference for eicosanoids since they can get through the membrane anyhow. For the cytokines we only have transmembrane receptors which won't work/be accessible in bacteria I think. - Jonas 22:46, 26 May 2008 (UTC)
- Sensing is indeed a problem. For example, expression of GPCRs is toxic for the bacterial membrane (see http://linkinghub.elsevier.com/retrieve/pii/S0167779904003348). A nuclear receptor (NR) would probably be more appropriate (plus direct activation of transcription indeed). The question then is whether we can find an appropriate eicosanoid/NR combination suitable for Crohn's or whether we can engineer/evolve an NR for an eicosanoid of our choice (e.g., Thromboxane B2) (probably a lot more challenging). --Y
- Prostaglandin E2 seems to target also nuclear receptors: Liver Receptor Homologue-1 LRH-1 (HUGO: NR5A2), which itself activates transcription of aromatase (http://cancerres.aacrjournals.org/cgi/content/abstract/65/2/657) + NR4A2 (NURR1), which binds to the NBRE response element - a construct expressing a reporter through PGE2->NR4A2->3xNBRE->reporter has been demonstrated in a colon cancer line (http://www.jbc.org/cgi/content/full/281/5/2676).
- There are still other possibilities though. The [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WSN-4GVGFB2-J&_user=877992&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000047079&_version=1&_urlVersion=0&_userid=877992&md5=fd4cf9f61ff8a745daaf2cfe39e4d32d PhoP - PhoQ histidine kinase system of S. typhimurium] should be able to detect antimicrobial peptides produced by the immune system and direct gene expression changes. Also, the [http://www.sciencemag.org/cgi/content/full/309/5735/774 OprF outer membrane protein of P. aeruginosa] can detect IFN-gamma (for these 2 cases we do need a gram-negative bacterium). Another even more exotic option could be to engineer a bacterial AHL receptor to recognize our eicosanoids. It should be feasible since AHL's and eicosanoids are similar lipid-derived molecules. Only thing is, that this is probably not the 3-month-time kind of feasible, especially if we don't want cross-reactions. (though it would be extremely fancy) :) - Jonas 19:56, 27 May 2008 (UTC)
- Are you sure those nuclear receptors bind PGE2? NR4A2 and NR5A2 are just upregulated when cells are treated with PGE2, the signaling still happens through GPCRs. PS: if we use nuclear receptors, we'll still have to fuse them to a transcription activator domain of a prokaryotic transcription factor in order to couple NR binding to RNA polymerase recruitment. - Jonas 16:38, 28 May 2008 (UTC)
- I think you are right: NR4A2 and NR5A2 seem to be ligand-independent nuclear receptors. So the activation probably happens through some kind of GPCR/GPCR-receptor mechanism :-( --Y
- I'd go through some literature if it wasn't for these damned exams :) If we don't find an eicosanoid/NR combination that works, we'll explore some more options. I feel like it can be done. IFN-gamma is probably produced, so the OprF system should work. It should be easy to implement as well. Don't know what the PhoP - PhoQ system exactly detects but it could be a very good option as well and also feasible to implement without too much tinkering. I could also take a quick look at an AHL receptor or so after the exams, maybe see if things can easily be modified, try a quick docking simulation, but let's keep that as an extra fun option. - Jonas 23:12, 28 May 2008 (UTC)
- Another thing, does anyone know how the IL10 in the L. lactis system is produced? I mean, is it exported, did they add an export signal sequence and does it fold correctly in the lumen? Or do they just let the IL 10 pile up in the cytoplasm and wait for the cell to die and release it into the surroundings? Or ... - Jonas 23:12, 28 May 2008 (UTC)
- PMID 12808464: "The hIL10 gene is synthetic—codon-optimized for L. lactis (see PMID 7590320) and fused to the usp45 secretion leader (PMID 2123812)." - usp45 encodes a secreted protein from Lactococcus lactis subsp. lactis MG1363. (PMID 2123812) --Ingethijs 12:06, 29 May 2008 (UTC)
- Exellent news. Alright, so if we're still looking for a NR, it'll have to be one of [http://en.wikipedia.org/wiki/Peroxisome_proliferator-activated_receptor these]. Haven't looked into it any further yet, but if there is anything, we should find it in this class. - Jonas 16:38, 29 May 2008 (UTC)
- "Endogenous ligands for the PPARs include free fatty acids and eicosanoids. PPARγ is activated by PGJ2 (a prostaglandin). In contrast, PPARα is activated by leukotriene B4." - from Wikipedia (PPAR) --Y
- Leukotriene B4 does not look too promising (http://www.ncbi.nlm.nih.gov/pubmed/8975956) --Y
- Hehe, wouldn't you just love science. Got an [http://www.ncbi.nlm.nih.gov/pubmed/17230539?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum article] from 2007 that says exactly the opposite. Plus it's even implicated in Crohn's if I'm not mistaking. We just might have our NR here. :) - Jonas 23:12, 29 May 2008 (UTC)
- Thing is however, that we'll need a [http://en.wikipedia.org/wiki/RXR Retinoid X Receptor] as well. And it will have to be activated by its [http://en.wikipedia.org/wiki/9-cis_retinoic_acid ligand 9-cis retinoic acid]. Or maybe a constitutive active receptor might work, don't know if there are mutants of the RXR. We've still got the bacterial systems though, so multiple options. - Jonas 07:53, 30 May 2008 (UTC)
- Got another idea for the sensor part. We could sense the heightened temperature that's associated with inflammation. This type of sensing would be fast and doesn't depend upon the production, stability or mobility of any kind of diffusible factor. A sensor like that could be a temperature sensitive riboswitch or small regulatory RNA like [http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2958.2007.05794.x this]. It might be a bit leaky but it should do the trick. PS: did I mention it would be relatively easy to implement, easy to assay, cheap to test etc. (Plus regulatory RNAs are fancy) :P - Jonas 17:54, 1 June 2008 (UTC)
- The Valencia team is doing something with temperature this year (Teachers Workshop) -Ingethijs 14:23, 2 June 2008 (UTC)
- Any idea whether they were planning on using RNA or protein-based sensors? - Jonas 16:32, 2 June 2008 (UTC) --> don't know but I guess RNA, and if so, probably for the same reasons you mentioned ;) -Ingethijs 17:46, 2 June 2008 (UTC)
- Damn them :) Ah well, we could still have one temperature sensor modulate our sigal or so. - Jonas 18:33, 2 June 2008 (UTC)
- Another variation on a theme :-): [http://www.ncbi.nlm.nih.gov/pubmed/12230973?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum by Cossart and coworkers] - Sigriddk 23:05, 9 June 2008 (UTC)
Proof of concept
- For a proof of concept, we would need to be able to set up such a system in vitro. The drug production could be "simulated" by replacing it by the production of GFP. We would then need a system for controling the level of the signaling protein (e.g., TNF-alpha cytokine). We can easily increase the level of the signaling protein by spiking in a fixed amount of it. But how do we decrease it? We cannot really have a model of the response of the gut to the drug. Maybe we could precipitate the signaling protein with an antibody? And how do we measure the concentration? By fluorescently labeling the signaling protein? --Y
- Another setup (probably much easier) could be to
- Grow the bacteria on a dish
- Remove them from the original medium
- Put them in a medium with a high concentration of signaling protein (i.e., TNF-alpha)
- Observe that production of GFP SLOWLY ramps up (maybe up to some maximum threshold to avoid overdosing)
- Put the bacteria back into a medium with a low or zero concentration of signaling protein
- Observe that GFP production is turned off slowly --Y
- As an easier, intermediate goal, we could simply have a sensing system that directly turns on the reporter (that would be like a proportional controller).
Planning
maybe we can start thinking about a nice planning (like Inge said). I was thinking of a schedule of intermediate goals to accomplish:
- open loop proportional controller (jonas said it is quite easy to construct because the proportional behaviour is already there)
- closed loop proportional controller: close the loop and make the bacteria sense the results of its actions
- add an integral controller: only need extra accumulator function, the other functions are shared with the proportional controller
The point of this schedule is that it's build gradually, but it doesn't include a modular build-up. And that will also be needed to assure that the project doesn't critically depend on the succes of a particular module. Some idea's for that? --nick
A fourth idea: localised 'evolution'
Based upon prof. Moureau's proposal during the first brainstorm session. I think it should be possible (at least theroretically, please comment on this) to do this just by using 3-4 'simple' components in a more complex system. (yes, there will be modeling :P )
- A type IIS or type III restriction endonuclease (to cut as far as possible from their recognition sequence, will probably be 25-30 nucleotides)
- A terminal deoxynucleotidyl transferase (TdT, adds nucleotides to nucleic acid ends) or similar
- A DNA ligase (ligates the ends)
- A DNA exonuclease if necessary (endogeneous exonuclease in the cell might already be high enough)
Thus, all basic molecular biology tools. Now for the system. The recognition sequence will have to be engineered into the gene to be under selection. This should be feasible for most proteins I think and would in most cases require us to adjust 2 amino acids or so. The site to be randomized will be the site where the endonuclease cuts.
A pulse of red light could start the system, it induces the cells to produce TdT and ligase (ligase maybe with a delay, we'd have to see how it turns out). When a certain level of TdT is reached, the cell should be induced to produce the endonuclease, but this induction should be immediately shut down again so only a few molecules are actually produced. The endonuclease cuts the site to be 'under evolution', exonuclease might nibble some nucleotides off but TdT, being high enough should add extra nucleotides as well. Maybe the TdT has to be brought into proximity by an interaction with the restriction endonuclease, Fos-Jun zipper stuff or so? The amount of nucleotides added/removed could be altered by changing system parameters. Ligase should peak shortly after and ligate the cut, maybe it will also have to be brought into proximity by protein interactions, no idea. Anyhow, when this is done, the system should shut itself down again, completely. In this way, for example a loop of a synthetic monoclonal antibody (or a surface protein to make things easier for us) is randomized in in a large population of cells, these cells can then be selected for binding to a fluorescently labeled antigen and be automatically sorted/enriched by FACS.
The advantage of working with the type IIS or type III endouclease instead of a Cre-LoxP system would be that recognition sites are small, they should not hinder normal function, and the process can be repeated by just flashing the red light again. Not too much nucleotides should be nibbled off though, but automatic 'randomization' of 14 amino acids would seem possible. It's just like mimicking VDJ-recombination in immune cells.
It seems possible to me to do this and I think it would require a lot of modeling as well. Also, the parts needed should be possible to find/work with. A system like this could be very valuable in for example binding site design and the affinity maturation of synthetic monoclonal antibodies, so we'd still be in the medical department.
At the moment I only see 2 things/problems which I can not estimate:
- Protecting the host from the restriction endonuclease while not protecting the gene to be randomized? Anyone know how do they clone these endonucleases?
- Finding the balance between the parts might be very tricky (or impossible, you tell me), and the efficiency might be low, which is compenated by sheer numbers and selection pressure though as plasmid loss = cell death.
Maybe the efficiency of the ligation could be increased by looping out the gene by inserting a lamda repressor site next to it on either side. -Jonas 21:04, 8 June 2008 (UTC)
A fifth idea: Timer module
I could imagine a timer module based upon the [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WK7-4DPBWPM-61&_user=877992&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000047079&_version=1&_urlVersion=0&_userid=877992&md5=b1333488579d3e005e4d81d0b977c737 hok/sok system] of plasmid maintenance. Where either the hok or the sok part is under the control of an inducible promotor. With hok it will have to be overactive outside the body while with sok it will have to be repressed. This could also be integrated in idea 3 or any other scheme that we come up with. - Jonas 08:26, 2 June 2008 (UTC)
A sixth idea: Topical medical application
I doubt whether a bug that is only used externally on humans would cause much less ethical issues, since they still end up in the environment. Anyhow, if the internal part of idea number three is the issue, we could do something like the following. Design a bug (use one already present on the human skin) that can produce a local anesthetic when induced by a flash of red light or something. Basic idea would be that you apply a cream with these bacteria on a sprained ankle or so, flash it and it starts to produce the anesthetic. You could also make the production temperature dependant, so when the heat and swelling go away, the production is decreased. So it's basically the same system as in the third idea. Anyhow, just crossed my mind, thought I'd spill it here before it's gone again :) -Jonas
A seventh idea: The D of the PID to study stem cell development
During the brainstorm session of 10/06 we discussed the biological implementation of a differentiator. A differentiator gets a concentration of a specific regulatory protein as an input signal, and outputs a signal (again a concentration, e.g. of GFP) proportional to the derivative of the input signal. This means that we could see whether the concentration of the protein under study increases or decreases. Moreover, if this system is implemented for several different proteins, which have their own associated color, we could see which regulator appears first, and this could give us an idea of the regulatory cascade responsible for stem cell differentiation. -Liesbeth
Mechanism
I don't know if anything has already been said about the realisation of this system but I think one way it could be done is like this:
It's a very nice idea by the way, not only useful for looking at cell differentiation, but also drug design, disease effects, ... The possibilities for a system like this seem endless. -Jonas 17:30, 16 June 2008 (UTC)
Brainstorm June 10th
(under construction -Ingethijs 13:30, 25 June 2008 (UTC))
Brainstorm June 17th
(under construction -Ingethijs 13:30, 25 June 2008 (UTC))
We continued again on the PID - D track...
Most confusion of last week resulted from the difference between what P, I and D do seperately, and what a PID controller does. In a PID controller, the nature of the input and the output is the same. eg. disease and resulting disease after the system has worked - the system produces the drug, but according to the difference between the output and the input. What we had in mind rather, is one system (bacteria) in which the disease marker is the input and the drug the output.
Based on last meeting, I figured a differentiator could be built like this:
it is based on a regulator that activates a sRNA that inhibits the translation of the regulator mRNA - the regulator mRNA closely follows the input signal
this way, according to the excess of sRNA or regulator mRNA, a different output will be produced
It is a very cool idea, but the way to colour this idea biologically, is not trivial...
So, we started again from the suggestions and ideas that were already here on the wiki. These key words may lead us to a project:
- bacteria – for the ease of manipulation
- modularity – not all eggs in one basket + benefit from the size of our team, so split up the project
- medical problem – seemed to be the major interest in the team
With these we propose the following framework (based on the example of eating chocolate when you have fever to generate a pulse of anti-fever stuff in the bacteria, and, when you eat no chocolate for a week (??), the bacteria decides to kill itself):
- make a sensor to an interesting molecule – trigger
- generate a pulse of expression
- construct a molecular timer
This way, we can subdivide the team:
Parts 1 and 2 could/should be feasible ánd , with a good choice of input/output, funny, interesting, crazy … (medical application, bioremediation,…), In part 1 and 2, the focus is on the application.
- Sensing chocolate, … Choosing a molecule /sensor combination that is bacterial and transfer it to E. coli is easier than choosing a eukaryotic one
- pulse expression generation – this could for instance be done with negative autoregulation. This autorepressor is then either directly coupled to an effector or regulator (operon), or can positively act on this effector or regulator (this would mean finding a dual regulator or adapting an activator such that it binds downstream of its promoter, thus inhibiting the passage of RNA polymerase). The effector could then be gfp, a drug, a regulatory sRNA, a regulator…
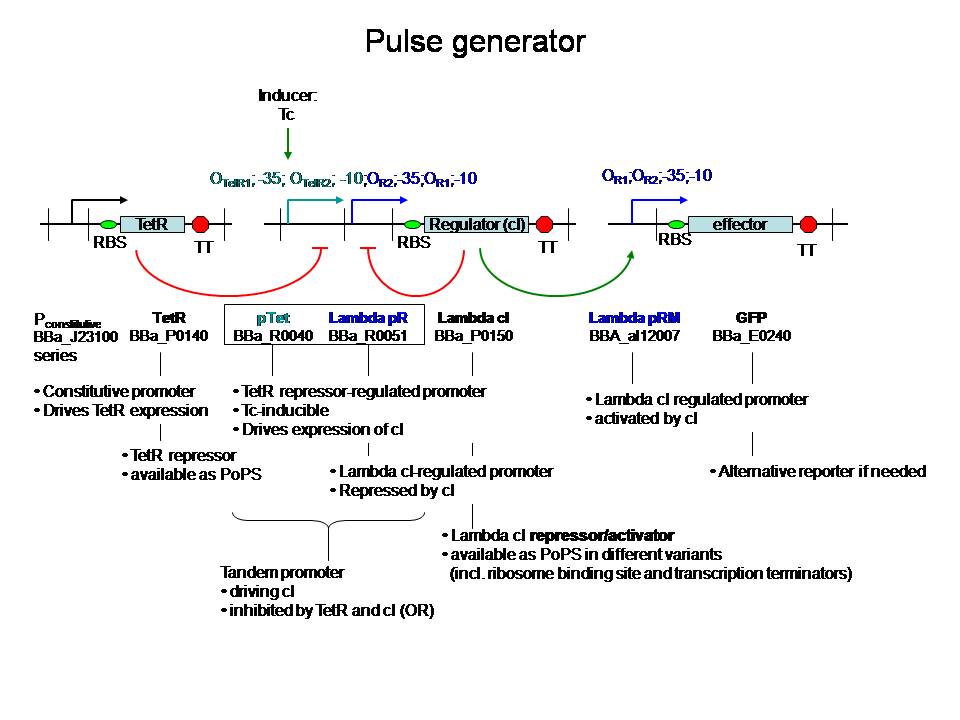
But, other pulse generators have been described before, eg. https://keaslinglab.lbl.gov/wiki/index.php?title=Synthetic_Biology_-_Devices_-_Pulse_Generator&printable=yes and Basu et al (2004, PNAS) Spatiotemporal control of gene expression with pulse-generating networks.
Part 3 is more challenging, but the project won’t suffer from it if not completed. The goal here would be to kill the bacteria if they are not longer needed. A gene for cell death could be switched on, or an essential gene could be switched off when no consistent signal is sensed within a certain period of time. “Consistent” signal could for instance be a signal that over some time reaches a certain threshold. Is this some kind of integration? If yes, it maybe is still possible to use it in the framework of a PID controller :)
In general, a timer depends on cascades - delays, but these go fast. For a slower timer, we probably have to focus on slowly degrading components, eg. HSL, and let their level have an effect
Brainstorm June 23rd
(under construction -Ingethijs 13:30, 25 June 2008 (UTC))
PID control - back to basics
The goal was to leave the confusion about input-output and the disease-drug system behind and look at each component of the PID controller separately.
- The P component produces an output that closely follows the input (proportional) - fast degrading protein
- the I component produces an output that 'memorizes' the input over a certain time period (integration) - slowly degrading component
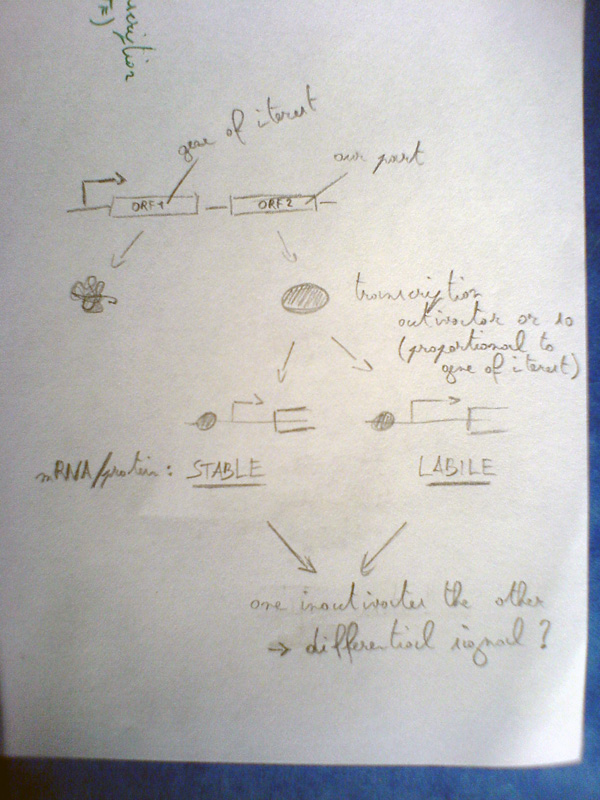
- The D component produces an output when the input changes (differentiation) - regulator/sRNA (would be fancy to work with sRNAs!) - the proposal of Jonas - JR had another proposal, building on the one of Jonas:
- "STABLE": a stable and fast folding GFP (or another effector) that contains a TEV protease site (in a harmless loop)
- "UNSTABLE": an unstable TEV protease that can specifically inactivate/degrade the GFP
This kind of differentiator can detect changes (no difference between increase and decrease). The rbs of the TEV should not be too strong - model! Good point, there's no TEV in the BioBricks yet. For inspiration: http://www.ncbi.nlm.nih.gov/pubmed/18476830
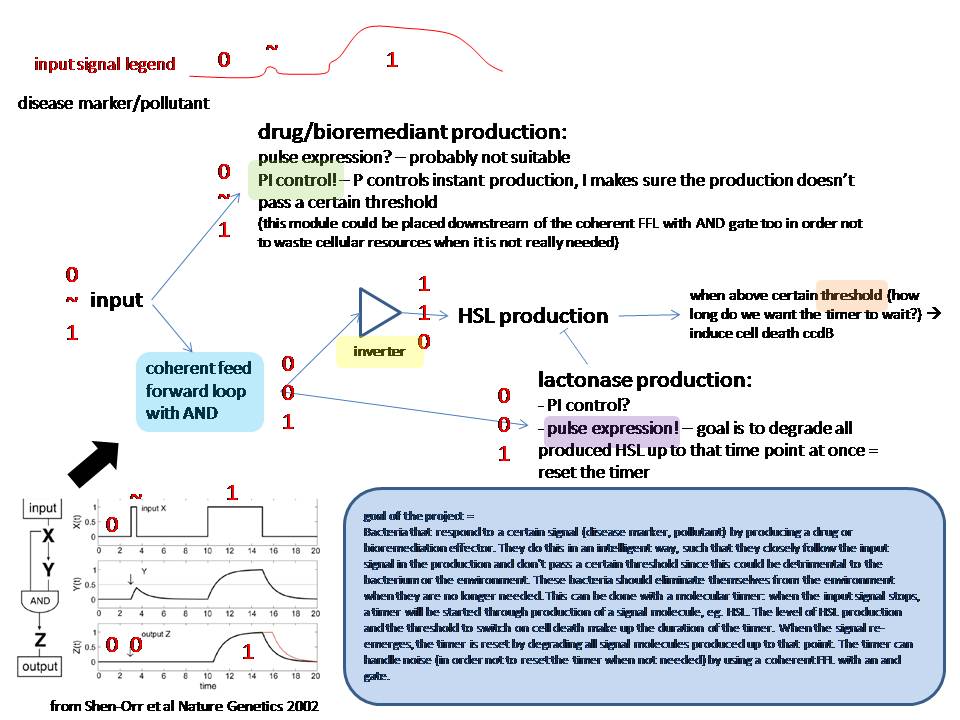
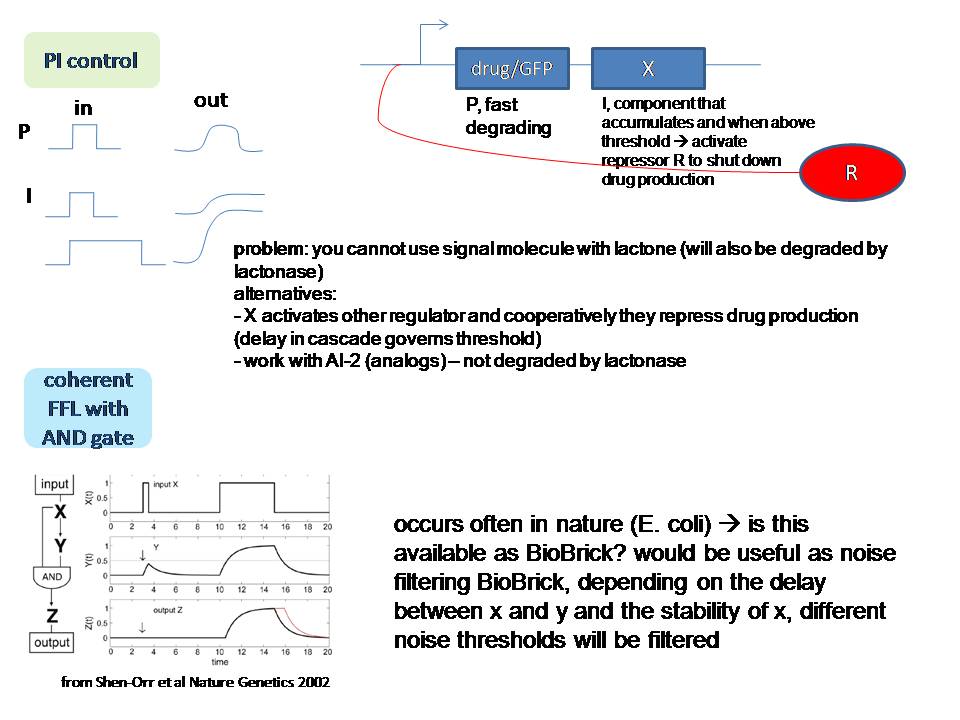
P and I are useful for the drug story: P controls the drug production and with I we can control the maximum amount of drugs that can be produced
Molecular timer
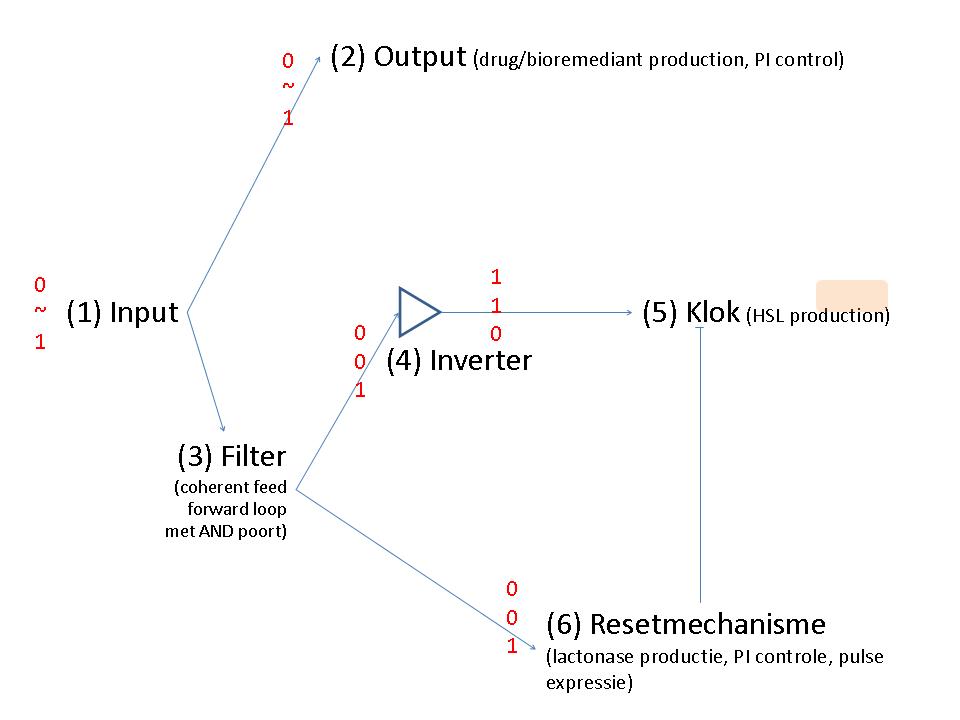
Regarding the "consistent signal": the coherent feedforward loop with an AND gate from Shen-orr's paper of 2002(PMID: 11967538) could be useful to filter the signal (have a look at figure 2 of the original paper).
When there is no input signal anymore, a signal that induces cell death should be produced. When the input signal returns consistently, the cascade towards cell death should be stopped.
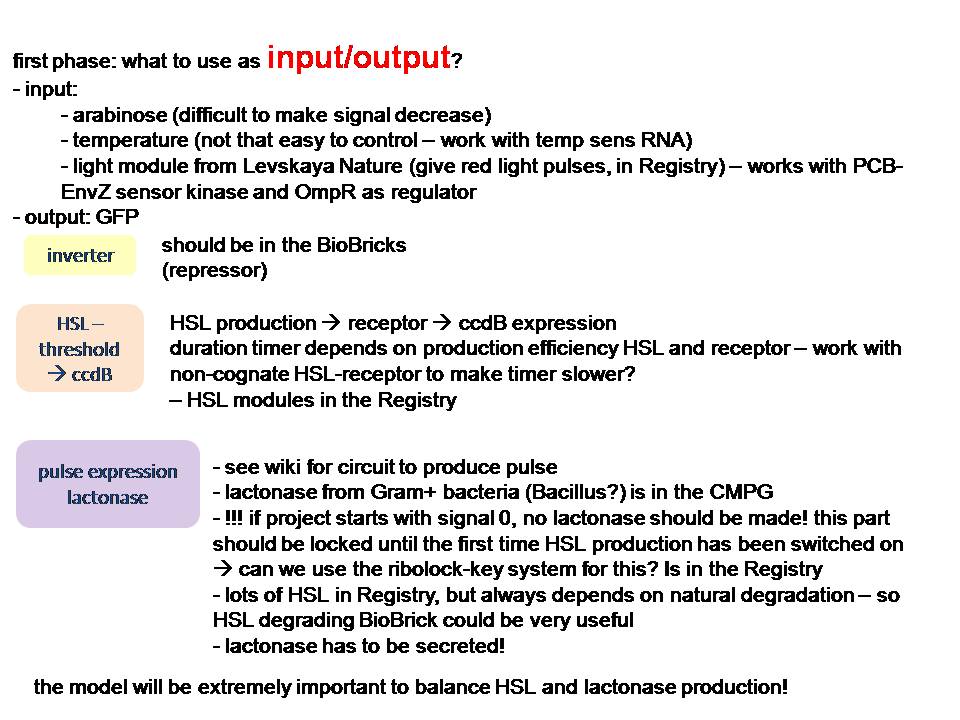
HSL production could be used to 'time' and lactonase production to reset the timer. Lactonase production has to be locked until the first time HSL has been produced.
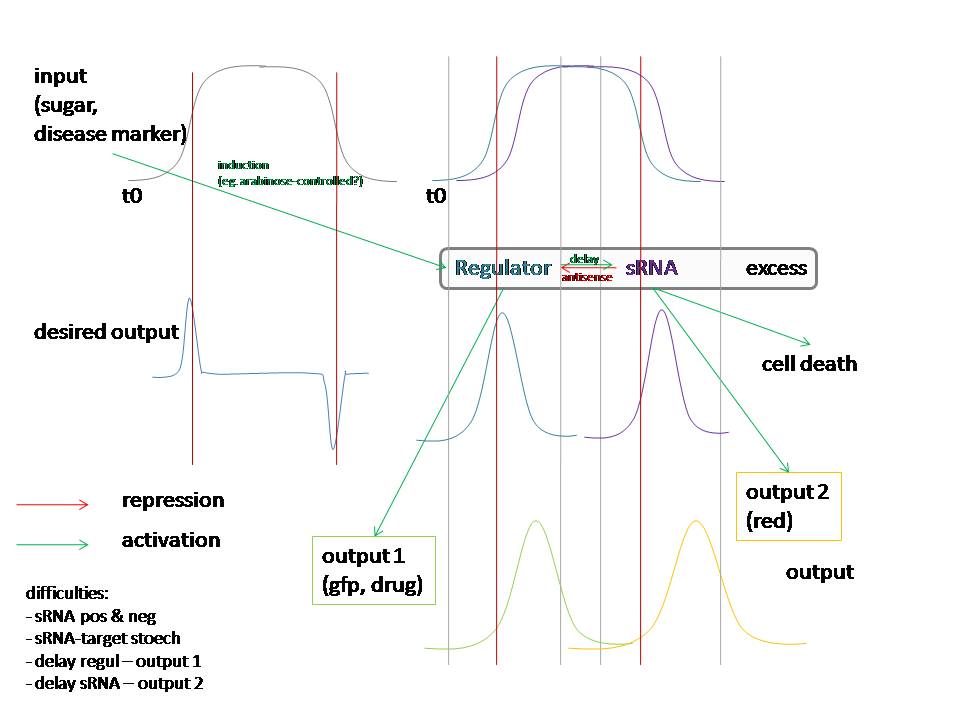
Proposal: see the figure too!
goal of the project =
Bacteria that respond to a certain signal (disease marker, pollutant) by producing a drug or bioremediation effector. They do this in an intelligent way, such that they closely follow the input signal in the production and don’t pass a certain threshold since this could be detrimental to the bacterium or the environment. These bacteria should eliminate themselves from the environment when they are no longer needed. This can be done with a molecular timer: when the input signal stops, a timer will be started through production of a signal molecule, eg. HSL. The level of HSL production and the threshold to switch on cell death make up the duration of the timer. When the signal re-emerges, the timer is reset by degrading all signal molecules produced up to that point. The timer can handle noise (in order not to reset the timer when not needed) by using a coherent FFL with an and gate.
Admittedly, this is also a hugely ambitious project... We could decide to do some part(s) of it :) Anyway, a proposition that involves circuit design is probably the most interesting from modeling perspective (although I have to admit I'm not familiar with docking modeling for instance - Inge).
Regarding the PI control of drug production in the image "timer part 3": I just had a look at [http://parts.mit.edu/igem07/index.php/NYMU_Taipei/ProjectDescription Taipei 2007], and they also "measured" insulin production by placing CinR+HSL in the same operon as insulin. If we make the timer module with lactonase, however, we cannot use this system. -Ingethijs 12:22, 26 June 2008 (UTC)
Other idea:
part Para->lacI
part Para->OlacI-|GFP
When arabinose is added, LacI is produced and this will inhibit GFP expression. Lactose can then be added to gradually titrate LacI and induce GFP expression. This system could be used as a "timer" since the production of GFP can be prepared, but will only start when lactose is added.
New ideas
- force E. coli into different modes by using 5 different sigma factors
Practical
- which E. coli to use?
Project
Input
Output
Filter
Inverter
Clock
Reset mechanism
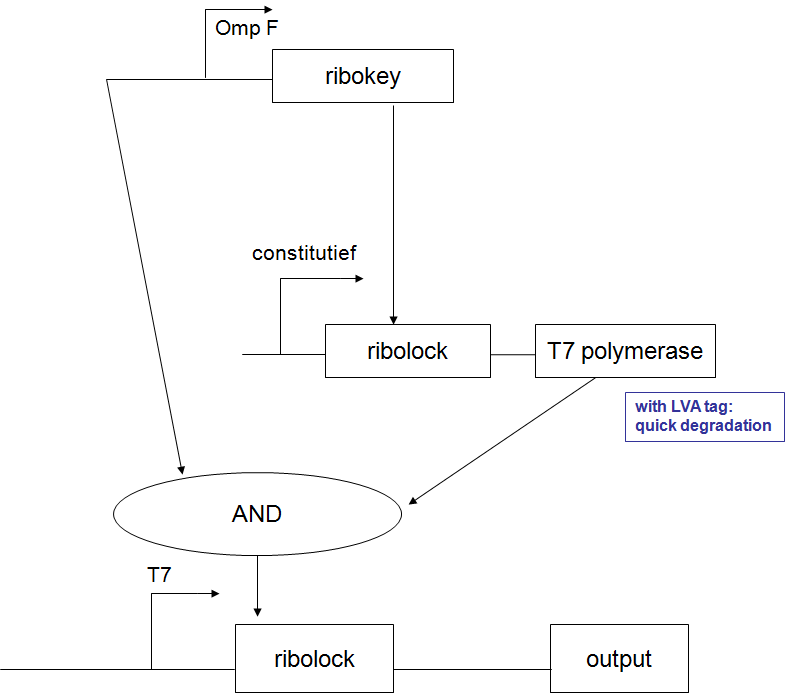
pulsexpressie van lactonase:
van Johan Robben - 7 juli:
een pulsgenerator met beschikbare BioBricks
De regulatorische delen zijn zo gekozen dat ze niet interfereren met andere genen in coli. Voor zover ik kan zien is het gebruik van een tandem-promoter een nieuwigheid, en misschien op zich al het proberen waard. 't Zou nog leuker zijn natuurlijk als ze verbeteringen kunnen aanbrengen.
Fijn hoe het hier in de details zit. Het gebruik van de partiele PR en PRM met dus omgekeerde OR1 en OR2 waardoor cI productie gerepressed wordt voordat de concentratie hoog genoeg wordt om effector transcriptie te initieren. Het enige waar ik mee zit is de efficientie van de transcriptie van cI vanaf pTet wanneer er cI op PR gebonden is. Dit kan misschien doorlezen van het RNA polymerase belemmeren maar zal waarschijnlijk wel ok zijn aangezien de concentratie cI voldoende laag gehouden wordt. In ieder geval, me like -Jonas
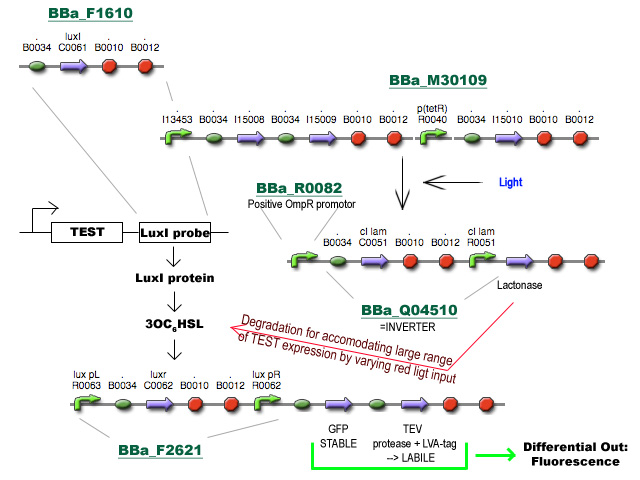
Project D
Zoals gevraagd, een concrete blik op Project D:
Ik ben vertrokken van het voorstel van Prof. Robben en heb nog een stukje toegevoegd om tegemoet te komen aan de brede range van expressieniveaus in de cel. Het gebruik van HSL synthese en degradatie (vanuit andere voorstellen) zorgt respectievelijk voor amplificatie van het signaal van differentiele regulatie en voor het verhogen van de dynamic range van expressieniveaus die het systeem aankan via belichting met rood licht op verschillende intensiteiten. Enfin, I had fun. Gooi er maar vragen/opmerkingen onder :)
(Begin linksboven) -Jonas 17:13, 5 July 2008 (UTC)
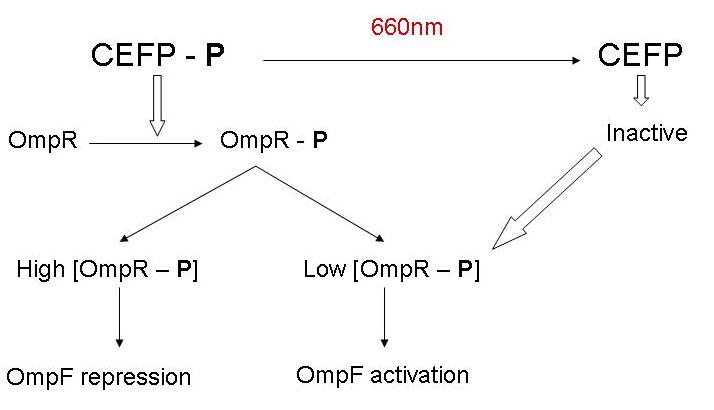
- Ik zie dat je gebruikt maakt van de pBAD promoter en de OmpR regulator. Indien we dit willen integreren met het project hierboven, zal ofwel deze stap, ofwel de input andere vorm moeten aannemen, want beide werken met de OmpR regulator. - Bmoeyaert
- Kan je eventueel een stroomschema maken van wat er juist "gebeurt", want ik kan het schema maar moeilijk volgen (en ik denk dat ik de enige niet ben). - Bmoeyaert
Ik zal in de loop van de dag een beetje een flow-chart proberen te maken. Zal hier voorlopig nu een korte beschrijving proberen te geven.
- BBa_F1610 fungeert als probe om het expressieniveau van een TEST gen te volgen. Deze BioBrick wordt achter het TEST gen geïnsereerd (vóór de transcriptie terminators, misschien zijn die van BBa_F1610 niet eens nodig) en zal vertaald worden tot LuxI proteine.
- LuxI produceert 3-oxohexanoyl-homoserine lactone (3OC6HSL) wat gesensed wordt via LuxR, geproduceerd door BioBrick BBa_F2621.
- De aanwezigheid van LuxR met gebonden 3OC6HSL fungeert als transcriptie activator vanaf de Lux pR promotor en initieert transcriptie van een stabiel en snel vouwend Green Fluorescent Protein met TEV herkenningssequentie en een labiel TEV protease. (met de sterkte van de Ribosome Binding Sites zal nog gespeeld moeten worden)
- TEV degradeert het GFP en zorgt voor een differentieel fluorescent signaal dat uitgelezen kan worden.
- Het stuk rechtsboven is basically een regelbare cascade die uitmondt in de productie van Lactonase wat de 3OC6HSL geproduceerd door LuxI afbreekt. Door de hoeveelheid lactonase onder fijne gegradeerde regulatie te plaatsen (hier nu bvb licht genomen) wordt de range van mogelijke expressieniveaus waarmee het systeem overweg kan uitgebreid.
- Al wat BBa_M30109 doet is lichtsensor produceren onder controle van pBAD en pTet. In het donker zorgt deze BioBrick voor transcriptie vanaf de OmpR promotor(BBa_R0082). Dit signaal gaat door de inverter BBa_Q04510 die output geeft via lactonase. In het donker wordt er dus geen extra lactonase tot expressie gebracht.
- Als het gebruik van pBAD en pOmpR lastig is kan lactonase simpelweg onder pLac (BBa_I14032) controle geplaatst worden, waar de hoeveelheid lactonase gecontroleerd kan worden door het toevoegen van IPTG. Heel het stuk rechtsboven kan dus eigenlijk ook door 1 enkele part vervangen worden onder de vorm van pLac-RBS-lactonase-STOP ;) -Jonas
PS: If the D is to be integrated in the PI project (mind the pun :P ) after the output-gene for example, the lactonase will have to go and a different HSL synthesis-sensor system will have to be used (examples are in the registry, minimal cross-reaction is a requirement)
 "
"