Team:Hawaii/Notebook/2008-08-30
From 2008.igem.org
(Difference between revisions)
(New page: {{Team:Hawaii/Header}} = Things we did today = == Wetlab work == ===Construction of p+r and rereplacement of BB-pRL1383a MCS=== :<strong> Grace</strong> [[Image:083008REdigests.jpg|right|...) |
(→Construction of p+r and rereplacement of BB-pRL1383a MCS) |
||
| Line 7: | Line 7: | ||
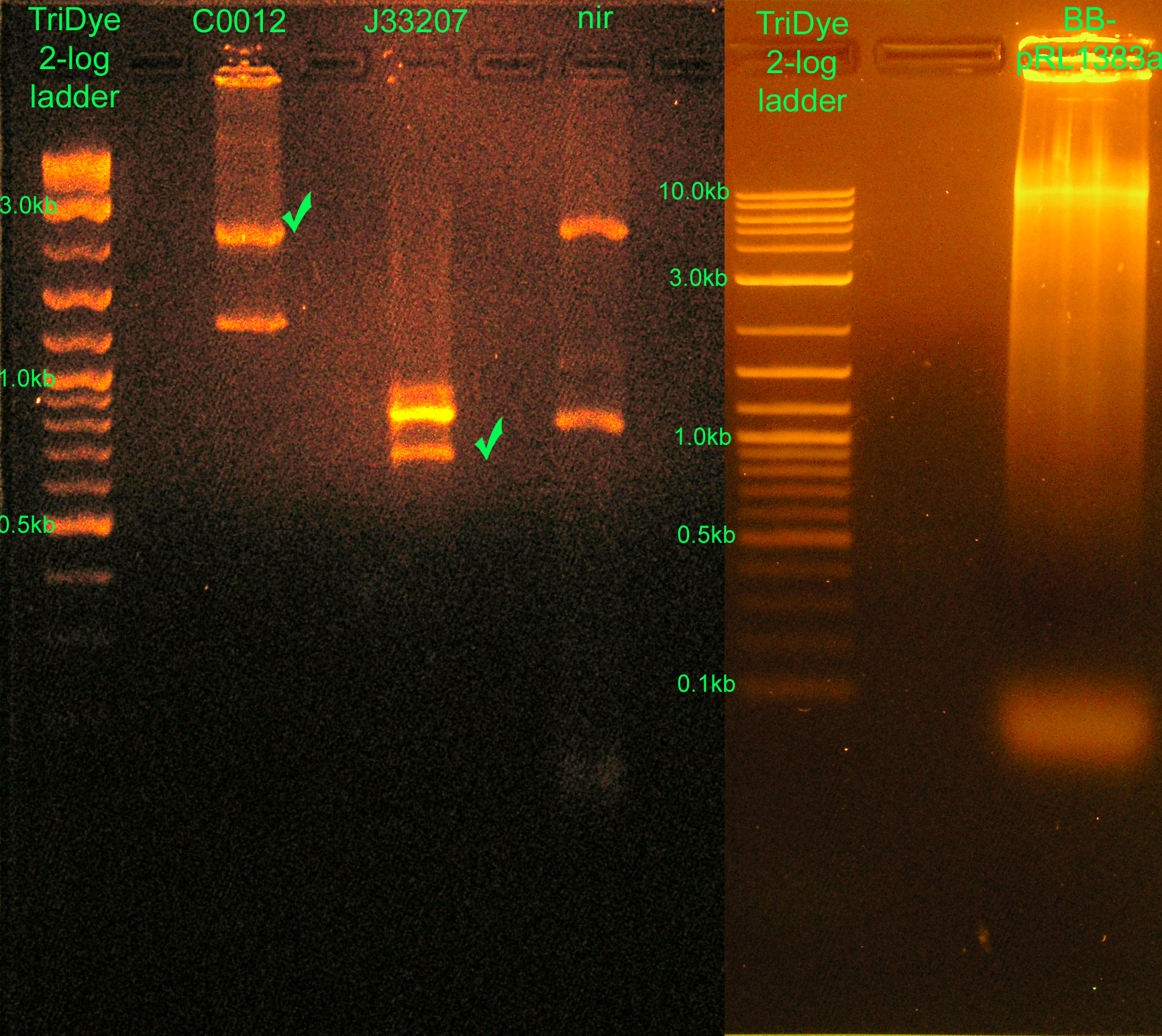
[[Image:083008REdigests.jpg|right|thumb|400px|EtBr stained 2% (L) and 0.8% (R) agarose gels ran at 60V for 2 hours and 1 hour, respectively. Thirty microliters of RE digest reaction were loaded into each well.]] | [[Image:083008REdigests.jpg|right|thumb|400px|EtBr stained 2% (L) and 0.8% (R) agarose gels ran at 60V for 2 hours and 1 hour, respectively. Thirty microliters of RE digest reaction were loaded into each well.]] | ||
:* Ran RE digests from last night on agarose gel | :* Ran RE digests from last night on agarose gel | ||
| + | ::* C0012: correct vector band ~2.1kb | ||
| + | ::* J33207: band at ~700bp (only one enzyme cut -- XbaI did not cut; PstI cuts because C0012 was cut by PstI) and ~850bp (uncut PCR product) | ||
| + | ::* nir: ~800bp band (from GFP contaminant in plasmid prep, cut only once) and ~2kb band (??) | ||
| + | ::* BB-pRL1383a: ran WAY too much product. Will redo RE digest with 20% of plasmid used. Since J33207 was only cut once, it's assumed BB-pRL1383a was cut only once as well. | ||
:* Extracted bands from gel | :* Extracted bands from gel | ||
:* Ligated: | :* Ligated: | ||
| - | |||
| - | |||
::* plac + rbs (B0034) | ::* plac + rbs (B0034) | ||
| - | :* RE digested p+r with EcoRI, XbaI, SpeI, PstI | + | :* PCR of J33207 |
| + | :* RE digested: | ||
| + | ::* p+r with EcoRI, XbaI, SpeI, PstI | ||
| + | ::* J33207 and BB-pRL1383a with EcoRI and PstI | ||
| + | :* Treated C0012 vector (pSB1A2) with SAP | ||
= Discussion = | = Discussion = | ||
Revision as of 23:52, 30 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Construction of p+r and rereplacement of BB-pRL1383a MCS
- Grace
- Ran RE digests from last night on agarose gel
- C0012: correct vector band ~2.1kb
- J33207: band at ~700bp (only one enzyme cut -- XbaI did not cut; PstI cuts because C0012 was cut by PstI) and ~850bp (uncut PCR product)
- nir: ~800bp band (from GFP contaminant in plasmid prep, cut only once) and ~2kb band (??)
- BB-pRL1383a: ran WAY too much product. Will redo RE digest with 20% of plasmid used. Since J33207 was only cut once, it's assumed BB-pRL1383a was cut only once as well.
- Extracted bands from gel
- Ligated:
- plac + rbs (B0034)
- PCR of J33207
- RE digested:
- p+r with EcoRI, XbaI, SpeI, PstI
- J33207 and BB-pRL1383a with EcoRI and PstI
- Treated C0012 vector (pSB1A2) with SAP
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"