Team:Bologna/Notebook
From 2008.igem.org
(Difference between revisions)
(→Week 3: from 08/04/08 to 08/10/08) |
(→Week 2: from 07/28/08 to 08/03/08) |
||
| Line 102: | Line 102: | ||
* Transformation and Amplification from our Lab Stock of [http://partsregistry.org/Part:BBa_S0100 S0100], [http://partsregistry.org/wiki/index.php?title=Part:BBa_I763005 I763005] and [http://partsregistry.org/Part:BBa_C0051 C0051] | * Transformation and Amplification from our Lab Stock of [http://partsregistry.org/Part:BBa_S0100 S0100], [http://partsregistry.org/wiki/index.php?title=Part:BBa_I763005 I763005] and [http://partsregistry.org/Part:BBa_C0051 C0051] | ||
| + | |||
| + | * Growth Curves of Dh5 Alpha, Top10 and XL1 Blue with Low Medium and High Copy Numbers to assay and define the different kinetics (Further Detail) | ||
= Week 3: from 08/04/08 to 08/10/08 = | = Week 3: from 08/04/08 to 08/10/08 = | ||
Revision as of 15:42, 23 October 2008
| HOME | PROJECT | TEAM | SUBMITTED PARTS | MODELING | WETLAB | LAB-BOOK | BIOSAFETY AND PROTOCOLS |
|---|
Under notebook are all relevant information regarding the work done every week in the laboratory and protocols followed.
Protocols
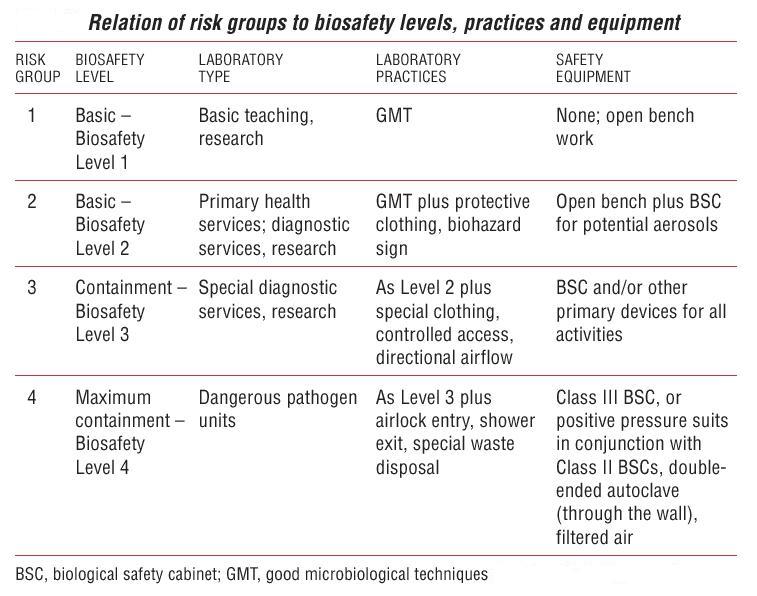
For more information, see Biosafety page
| Protocol | Biosafety level |
| Plates preparation | |
| Amplification | |
| Transformation | |
| Inoculation | |
| Miniprep | |
| Digestion reaction | |
| Gel preparation | |
| Electrophoretic run | |
| Gel extraction | |
| Ligation reaction | |
| Chemiocompetent cells | |
| Mediums and buffers | |
| Antibiotics stocks preparation | |
| IPTG stocks preparation | |
| Fluorescence test | |
| M9 supplemented media | |
Week 1: from 07/21/08 to 07/27/08
General Preparations
- Preparation of chemiocompetent cells from E. Coli DH5α, Top10 and DB 3.1
- Preparation of antibiotic stocks for Ampicillin and Kanamicin
- Preparation of LB medium and LB plates for cloning.
Week 2: from 07/28/08 to 08/03/08
- Eluition and Amplification from 2008 Registry Collection: [http://partsregistry.org/Part:BBa_R0082 R0082,] [http://partsregistry.org/Part:BBa_R0083, R0083], [http://partsregistry.org/wiki/index.php/Part:BBa_M30109 M30109] in TOP10 strain to build and characterize the Light response system to be our spatial selective trigger.
- Eluition and Amplification from 2008 Registry Collection: [http://partsregistry.org/Part:BBa_E0240 E0240], [http://partsregistry.org/Part:BBa_P1010 pSB3K3_P1010]in DB3.1 and the Practice Promoter Set ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J23103/ J23150, J23151, J23102]) to test and set up the new [http://partsregistry.org/Measurement Biobrick Standard Measurement Protocol]
- Transformation and Amplification from our Lab Stock of [http://partsregistry.org/Part:BBa_S0100 S0100], [http://partsregistry.org/wiki/index.php?title=Part:BBa_I763005 I763005] and [http://partsregistry.org/Part:BBa_C0051 C0051]
- Growth Curves of Dh5 Alpha, Top10 and XL1 Blue with Low Medium and High Copy Numbers to assay and define the different kinetics (Further Detail)
Week 3: from 08/04/08 to 08/10/08
- Digestion and Control Gel Run of the previous amplified constructs :
1. S0100 E/S
Consistent Part Length
2. PLAC-CI X/P
Consistent Part Length
3. R0083 S/P
Single Vector Band as Expexted. Is Hard to verify the Part length correctness given the small size
4. R0082 S/P
Single Vector Band as Expexted. Is Hard to verify the Part length correctness given the small size
5. C0051 X/P
Consistent Part Length.
7. M30105 E/S
The Part appears not consistent. The Gel has unexpected multiple bands.
8. RBS GFP TAG X/P
Consistent Part Length
9.Pλ GFP X/P
Consistent Part Length.
- Ligation of R0082 and R0083 with E0240 to obtain a Reporter for the Light Driven Trigger.
Week 4: from 08/11/08 to 08/17/08
Week 5: from 08/18/08 to 08/24/08
Week 6: from 08/25/08 to 08/31/08
Week 7: from 09/01/08 to 09/07/08
Week 8: from 09/08/08 to 09/14/08
Week 9: from 09/15/08 to 09/21/08
Week 10: from 09/22/08 to 09/28/08
Week 11: from 09/29/08 to 10/05/08
Week 12: from 10/06/08 to 10/12/08
Week 13: from 10/13/08 to 10/19/08
Week 14: from 10/20/08 to 10/26/08
Week 15: from 10/27/08 to 10/29/08
 "
"


