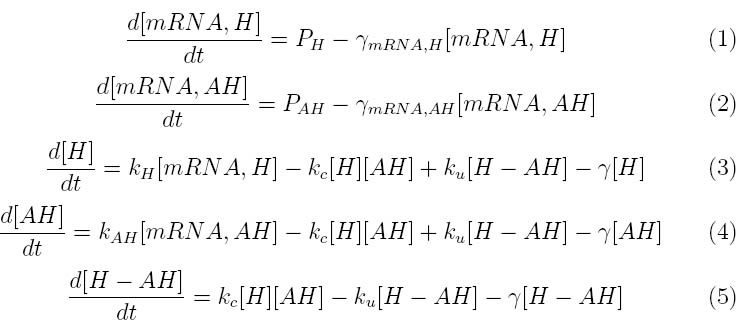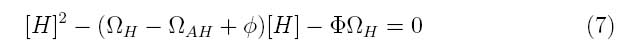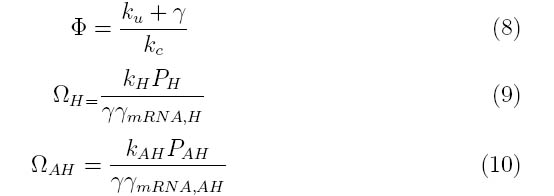Team:UC Berkeley/Modeling
From 2008.igem.org
ChrisBrown (Talk | contribs) |
ChrisBrown (Talk | contribs) |
||
| Line 15: | Line 15: | ||
Our holin-lysozyme lysis device consists of holin and lysozyme under an inducible promoter and antiholin under a constitutive promoter. For more information on our lysis device, click here [[https://2008.igem.org/Team:UC_Berkeley/LysisDevice]]. | Our holin-lysozyme lysis device consists of holin and lysozyme under an inducible promoter and antiholin under a constitutive promoter. For more information on our lysis device, click here [[https://2008.igem.org/Team:UC_Berkeley/LysisDevice]]. | ||
| - | The kinetics of our | + | The kinetics of our phage lysis device was modeled to help our team gain an insight into the behavior of our system. The below equations describe our system |
[[Image:cdb1.jpg]] | [[Image:cdb1.jpg]] | ||
| - | Where P1 and P2 represent the mRNA promoter strengths, | + | Where P1 and P2 represent the mRNA promoter strengths, γ_mRNA,H and γ_mRNA,AH are the degradation rates for holin and antiholin respectively and γ is the protein degradation rate, k,H , k,AH and k,c represent the rate constants for holin and antiholin formation and the coupling rate for the holin-antiholin dimer. |
====Transfer Function and Dimensionless Parameters==== | ====Transfer Function and Dimensionless Parameters==== | ||
| Line 37: | Line 37: | ||
These dimensionless numbers can assist in the optimization of lysis device design because they describe the important parameters in the system. | These dimensionless numbers can assist in the optimization of lysis device design because they describe the important parameters in the system. | ||
| - | For example, Ω_H consists of the rate constants for the formation of holin mRNA and the holin protein divided by their degradation rates. Strong promoters on holin will increase the value of Ω_H | + | For example, Ω_H consists of the rate constants for the formation of holin mRNA and the holin protein divided by their degradation rates. Strong promoters on holin will increase the value of Ω_H, while high protein degradation rates will decrease Ω_H. |
| - | + | Ω_AH describes the constants and degradation rates for anti-holin. | |
Φ describes the importance of the coupling and uncoupling rates of holin-antiholin dimer as well as the degradation rate of the dimer. | Φ describes the importance of the coupling and uncoupling rates of holin-antiholin dimer as well as the degradation rate of the dimer. | ||
====Graphs==== | ====Graphs==== | ||
| - | Physiologically relevant values for Ω_H, Ω_AH and Φ were estimated based on rate constants for similar proteins. This system was input into MatLab to produce the following graph. Since there is a degree of uncertainty in these estimates, the graph spans several orders of magnitude above and below our estimated values. | + | |
| + | Physiologically relevant values for Ω_H, Ω_AH and Φ were estimated based on rate constants for similar proteins. This system was input into MatLab to produce the following graph. Since there is a degree of uncertainty in these estimates, the graph spans several orders of magnitude above and below our estimated values. For the MatLab code used to produce these graphs, please click here [[html:Matlab code]]. | ||
[[Image:image1.png]] | [[Image:image1.png]] | ||
| Line 50: | Line 51: | ||
The literature indicates that at the time of lysis, cells infected with λ phage have approximately 1000 holin proteins. Therefore, the critical concentration of holin (Hc) was set at 1000 holin proteins per cell. | The literature indicates that at the time of lysis, cells infected with λ phage have approximately 1000 holin proteins. Therefore, the critical concentration of holin (Hc) was set at 1000 holin proteins per cell. | ||
| - | The horizontal line at y=1 represents the critical holin concentration needed induce lysis. As one would expect by looking at the dimensionless value for holin (omega H), as the strength of the holin promoter increases, the amount of holin at steady state increases. | + | The horizontal line at y=1 represents the critical holin concentration needed induce lysis. If the system produces more than the critical concentration of holin (>1000 free holin proteins), lysis is expected to occur. |
| + | |||
| + | As one would expect by looking at the dimensionless value for holin (omega H), as the strength of the holin promoter increases, the amount of holin at steady state increases. | ||
The graph also shows that the system is not very sensitive to small amounts of antiholin. Larger amounts of antiholin push the system equilibrium to the left and would require a stronger promoter on holin or a weaker binding interaction between holin and antiholin to reach critical concentration. This is supported by the observation that in a system with no antiholin, lysis can occur with holin under a weaker promoter. | The graph also shows that the system is not very sensitive to small amounts of antiholin. Larger amounts of antiholin push the system equilibrium to the left and would require a stronger promoter on holin or a weaker binding interaction between holin and antiholin to reach critical concentration. This is supported by the observation that in a system with no antiholin, lysis can occur with holin under a weaker promoter. | ||
Revision as of 04:08, 28 October 2008
Contents |
Introduction
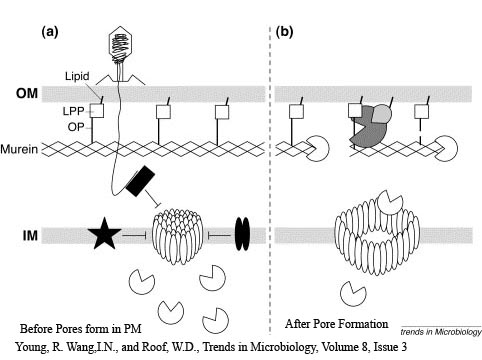
The holin-lysozyme phage lysis device consists of three protein;
Holin - inner membrane-bound proteins that when complexed with other holin proteins, create holes in the membrane that allow lysozyme access to the periplasm.
Antiholin - inner membrane-bound protein that binds to and inactivates holin to produce a holin-antiholin dimer. This dimer becomes active after holin forms enough pores in the plasma membrane to cause a loss of proton motive force between the periplasm and the cytosol.
Lysozyme - once holin forms pores in the inner membrane, lysozyme enters the periplasm and degrades peptidoglycan, resulting in cell lysis.
Governing Equations
Our holin-lysozyme lysis device consists of holin and lysozyme under an inducible promoter and antiholin under a constitutive promoter. For more information on our lysis device, click here [[1]].
The kinetics of our phage lysis device was modeled to help our team gain an insight into the behavior of our system. The below equations describe our system
Where P1 and P2 represent the mRNA promoter strengths, γ_mRNA,H and γ_mRNA,AH are the degradation rates for holin and antiholin respectively and γ is the protein degradation rate, k,H , k,AH and k,c represent the rate constants for holin and antiholin formation and the coupling rate for the holin-antiholin dimer.
Transfer Function and Dimensionless Parameters
At steady state,
By making this assumption, the system of equations can be simplified into the following transfer function
Where the system can be divided into three dimensionless parameters which describe the behavior of the holin-antiholin dimer and the holin and antiholin proteins.
These dimensionless numbers can assist in the optimization of lysis device design because they describe the important parameters in the system.
For example, Ω_H consists of the rate constants for the formation of holin mRNA and the holin protein divided by their degradation rates. Strong promoters on holin will increase the value of Ω_H, while high protein degradation rates will decrease Ω_H.
Ω_AH describes the constants and degradation rates for anti-holin.
Φ describes the importance of the coupling and uncoupling rates of holin-antiholin dimer as well as the degradation rate of the dimer.
Graphs
Physiologically relevant values for Ω_H, Ω_AH and Φ were estimated based on rate constants for similar proteins. This system was input into MatLab to produce the following graph. Since there is a degree of uncertainty in these estimates, the graph spans several orders of magnitude above and below our estimated values. For the MatLab code used to produce these graphs, please click here html:Matlab code.
The literature indicates that at the time of lysis, cells infected with λ phage have approximately 1000 holin proteins. Therefore, the critical concentration of holin (Hc) was set at 1000 holin proteins per cell.
The horizontal line at y=1 represents the critical holin concentration needed induce lysis. If the system produces more than the critical concentration of holin (>1000 free holin proteins), lysis is expected to occur.
As one would expect by looking at the dimensionless value for holin (omega H), as the strength of the holin promoter increases, the amount of holin at steady state increases.
The graph also shows that the system is not very sensitive to small amounts of antiholin. Larger amounts of antiholin push the system equilibrium to the left and would require a stronger promoter on holin or a weaker binding interaction between holin and antiholin to reach critical concentration. This is supported by the observation that in a system with no antiholin, lysis can occur with holin under a weaker promoter.
Varying Φ, the dimensionless parameter that describes the coupling and uncoupling behavior of the holin-antiholin dimer, reveals that when the binding of holin-antiholin is stronger, a stronger promoter is required to reach the critical holin concentration.
Conclusions
Since several phage systems and many promoters are currently in use, understanding the important parameters of the system allows one to choose appropriate promoters and phage proteins to optimize lysis behavior.
 "
"