Team:Freiburg Cloning Strategy
From 2008.igem.org
(Difference between revisions)
| Line 925: | Line 925: | ||
[[Image:Freiburg2008_TV_CMVRluc.jpg|700px]] | [[Image:Freiburg2008_TV_CMVRluc.jpg|700px]] | ||
<br> | <br> | ||
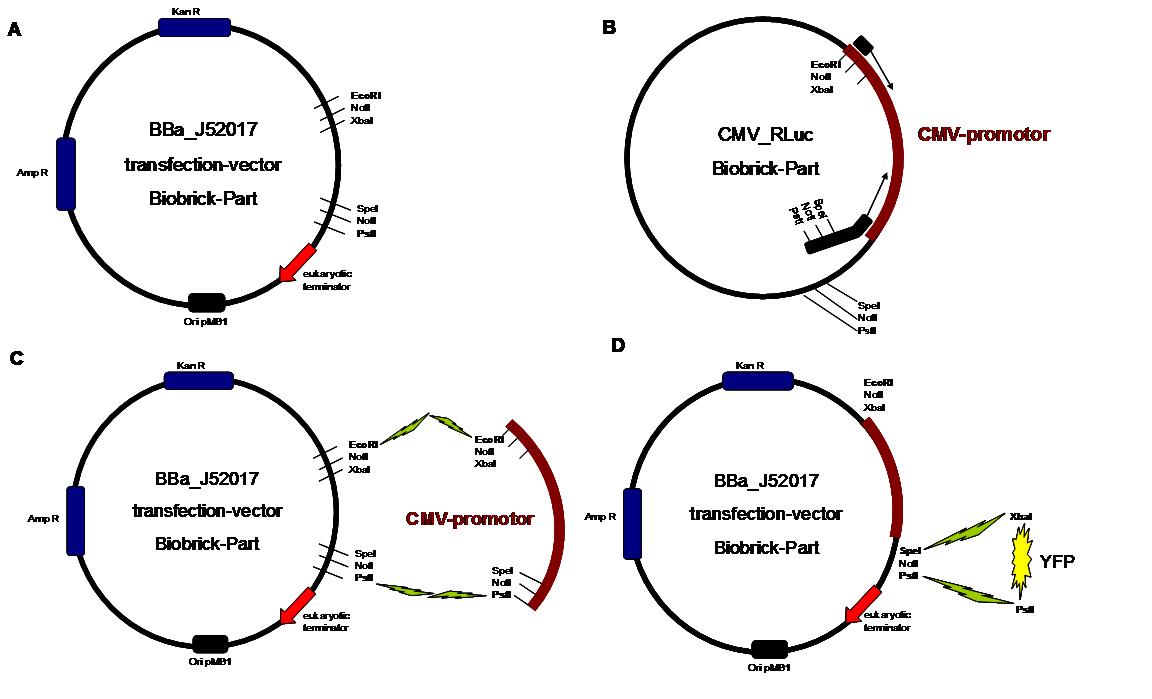
| - | '''Figure 1''' A shows the Biobrick part BBa_J52017. The transfectionvektor for eukaryotic cell systems has an ampicillin- and a kanamycin resistance cassette. The multiple cloning site contains the Biobrick standard restriction sites EcoRI, NotI, XbaI, SpeI, NotI, PstI followed by an eukaryotic terminator sequence. | + | '''Figure 1''' . '''A''' shows the Biobrick part BBa_J52017. The transfectionvektor for eukaryotic cell systems has an ampicillin- and a kanamycin resistance cassette. The multiple cloning site contains the Biobrick standard restriction sites EcoRI, NotI, XbaI, SpeI, NotI, PstI followed by an eukaryotic terminator sequence. '''B''' The CMV-promotor fragment was obtained by PCR with the Biobrick BBa-J52038 template. '''C''' The PCR product was cloned into the transfectionvector by EcoRI and PstI to get a final eukaryotic transfection-system. '''D''' To test the efficiency of expression a gene-fragment coding for the yellow fluorescent protein was put into the vector behind CMV-Promotor.<br> |
<br> | <br> | ||
[[Image:Freiburg2008_Konstrukte.jpg|700px]] | [[Image:Freiburg2008_Konstrukte.jpg|700px]] | ||
<br> | <br> | ||
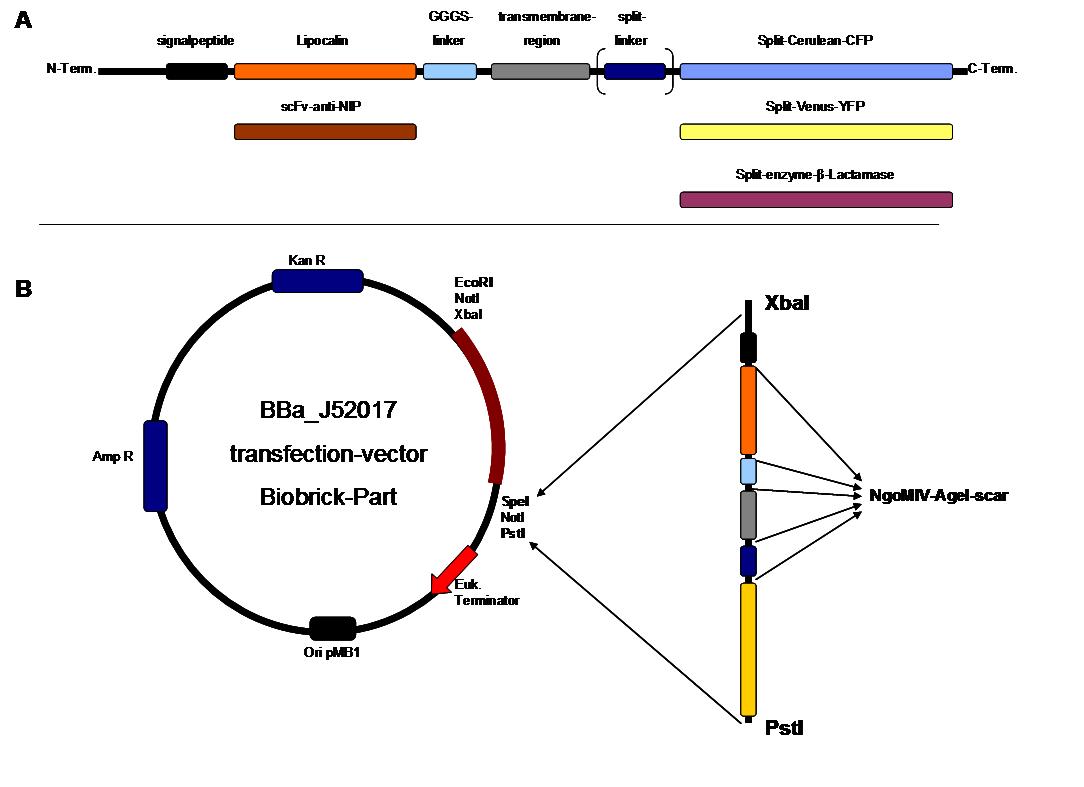
| + | '''Figure 2''' . '''A''' figure 1 A gives an overview about the cloning constructs. The N-terminal signal-peptide ensures protein transport to the cytoplasmamembrane. Lipocalin and the scFv-ani-NIP are the extracytoplasmatic parts of the construct to mediate signaltransduction into the cell. The GGGS-Linker keeps a distance to the transmembraneregion to overcome surface structures of the cell and to avoid a total inflexibility. Split fluorophor linker is only necessary for the C-terminal Split parts of Cerulean-CFP and Split-Venus-YFP. The Split enzymes β-Lactamese and Luciferase and the split-fluorophors CFP and YFP are the cytoplasmatic parts of the constructs. If there is a clustering of this synthetic receptor-system caused by the corresponding binding parts of Lipocalin and scFv-anti-NIP the split parts come together to create a functional protein, which allows a detection. | ||
| + | '''B''' The different constructions described in figure 2.A were cloned into the transfectionvector system by using the restriction sites XbaI and PstI to ensure a functional ATG-start codon which is part of the XbaI recognition-sequence in the iGEM-prefix<br> | ||
| + | <br> | ||
| + | |||
[[Image:Freiburg2008_Lipo+bla1+YFP.jpg|500px]] | [[Image:Freiburg2008_Lipo+bla1+YFP.jpg|500px]] | ||
<br> | <br> | ||
Revision as of 08:58, 28 October 2008
 "
"