Team:Freiburg Cloning Strategy
From 2008.igem.org
(Difference between revisions)
| Line 935: | Line 935: | ||
</table> | </table> | ||
<br><br><br> | <br><br><br> | ||
| - | '''METHODS''' | + | [['''METHODS''']]<br> |
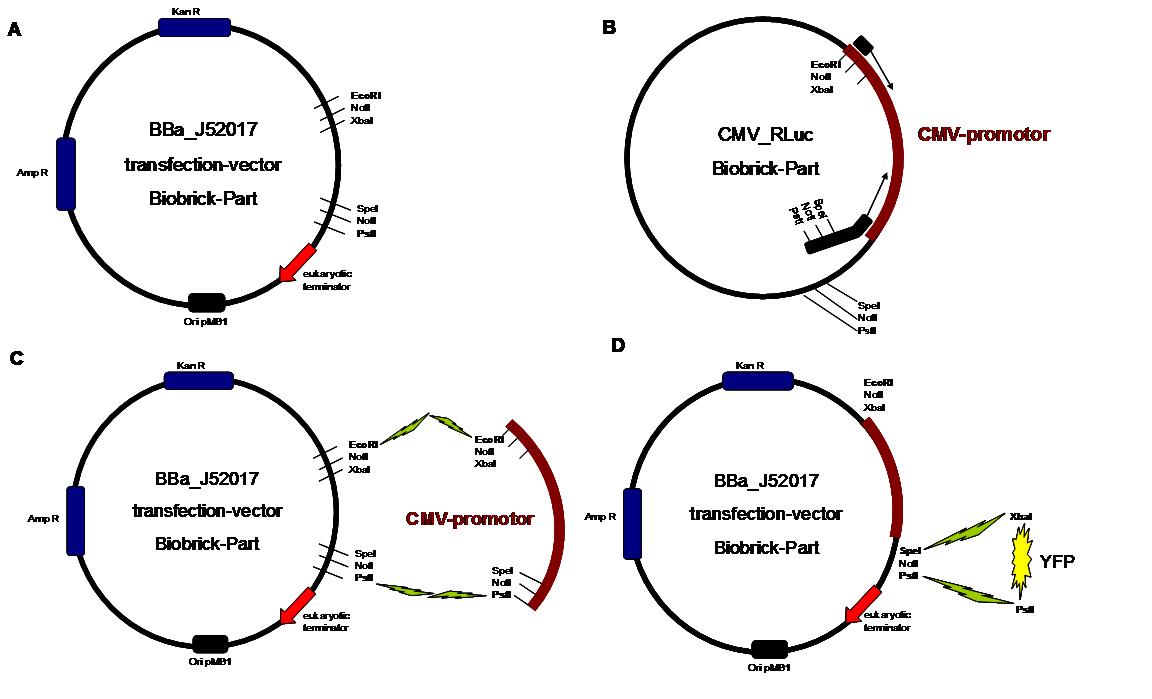
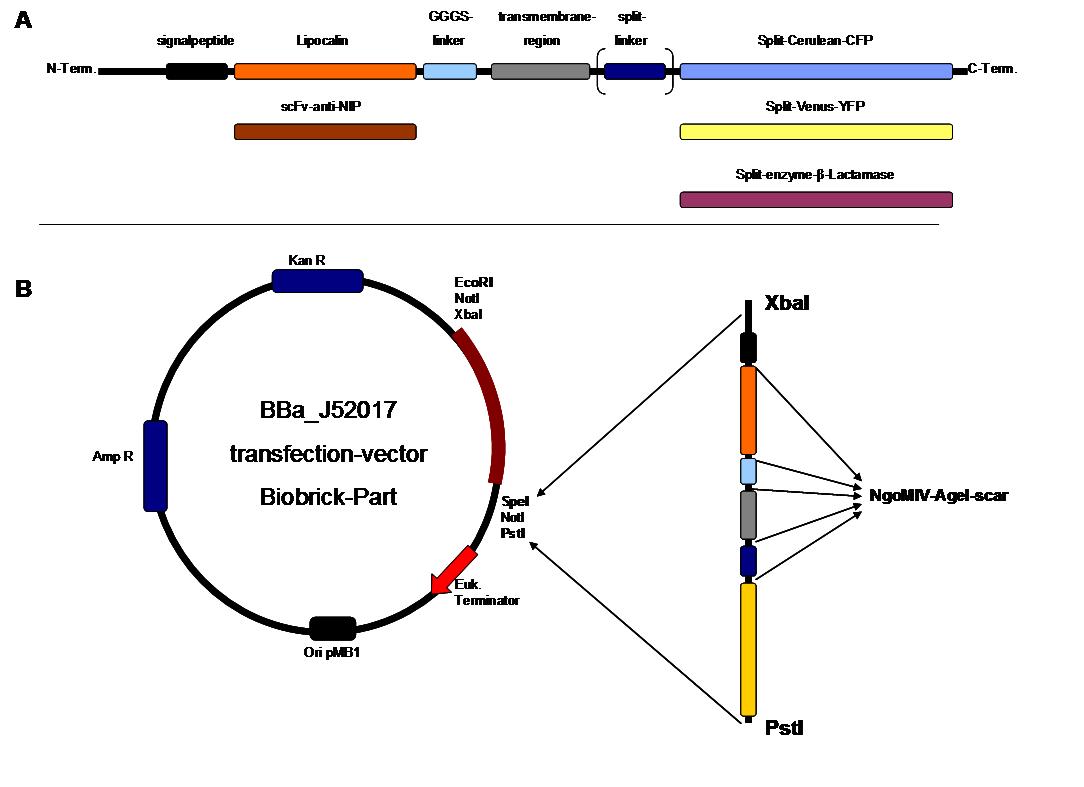
The cloning was started with a preparative digestion of the DNA-Plasmids. To clone fusion parts the vector constructs were digested with AgeI and PstI to open the Biobrick suffix. The inserts were digested with NgoMIV and PstI. For cloning into the transfection-vector the enzymes SpeI and PstI were used for vector and XbaI, PstI for insert to keep up the ATG-start codon in the XbaI restriction site of the biobrick suffix. All restriction-enzymes were ordered from New England Biolabs. After digestion the DNA-fragments were separated on a 1% agarose gel. The DNA-band of interest was isolated and purified with the QIAGEN QIAquick Gel Extraction Kit. For the ligation a 3 molar excess of the insert was put together with the vector-fragment and ligated with a Quick ligase (New England Biolabs). After half an our at room temperature the DNA was transformed to chemical competent E.coli strain XL1 cells, plated on 2YT-agar-plates and incubated at 37°C over night. After picking clones and growing in 5ml LB-medium, the plasmid DNA was isolated by QIAGEN QIAprep Spin Miniprep Kit. A test digestion was prepared with about 0,5µg Plasmid DNA and NotI restriction enzyme to isolate the fusion-protein from the vector and to control if the expected bands were obtained. After a positive result the clones were sent to GATC-Biotech for sequencing. | The cloning was started with a preparative digestion of the DNA-Plasmids. To clone fusion parts the vector constructs were digested with AgeI and PstI to open the Biobrick suffix. The inserts were digested with NgoMIV and PstI. For cloning into the transfection-vector the enzymes SpeI and PstI were used for vector and XbaI, PstI for insert to keep up the ATG-start codon in the XbaI restriction site of the biobrick suffix. All restriction-enzymes were ordered from New England Biolabs. After digestion the DNA-fragments were separated on a 1% agarose gel. The DNA-band of interest was isolated and purified with the QIAGEN QIAquick Gel Extraction Kit. For the ligation a 3 molar excess of the insert was put together with the vector-fragment and ligated with a Quick ligase (New England Biolabs). After half an our at room temperature the DNA was transformed to chemical competent E.coli strain XL1 cells, plated on 2YT-agar-plates and incubated at 37°C over night. After picking clones and growing in 5ml LB-medium, the plasmid DNA was isolated by QIAGEN QIAprep Spin Miniprep Kit. A test digestion was prepared with about 0,5µg Plasmid DNA and NotI restriction enzyme to isolate the fusion-protein from the vector and to control if the expected bands were obtained. After a positive result the clones were sent to GATC-Biotech for sequencing. | ||
The GGGS-Linker was produced by Klenow -fill-in-PCR. Two primers were designed align to each other at 60°C and filled to a complete dobble-strand by Klenow Polymerase fragment. | The GGGS-Linker was produced by Klenow -fill-in-PCR. Two primers were designed align to each other at 60°C and filled to a complete dobble-strand by Klenow Polymerase fragment. | ||
| + | <br> | ||
| + | Digestion Protocol <br> | ||
| + | - about 2µg Plasmid-Prep in 20µl <br> | ||
| + | - 2 µl NEB Buffer 10x<br> | ||
| + | - 1 µl NEB enzyme 1 (NgoMIV, AgeI, XbaI, EcoRI)<br> | ||
| + | - 1 µl NEB enzyme 2 (PstI, SpeI)<br> | ||
| + | - 0,2µl BSA 100x<br> | ||
| + | <br> | ||
| + | Ligation<br> | ||
| + | - 10µl volume of vector and insert DNA (about 50ng vector-DNA)<br> | ||
| + | - 1 µl DNA Quick Ligase (New England Biolabs)<br> | ||
| + | - 10 µl Quick Ligase Buffer<br> | ||
| + | <br> | ||
| + | Analytic digestion<br> | ||
| + | - about 0,5 µg Plasmid-DNA in 5µl<br> | ||
| + | - 5µl H2O<br> | ||
| + | - 0,5 µl NotI<br> | ||
| + | - 1µl NEB-Buffer<br> | ||
| + | - 0,1 µl BSA<br> | ||
| + | <br> | ||
| + | Transformation<br> | ||
| + | Competent cells (100µl) werde defrosted on ice<br> | ||
| + | 10µl of the ligation was added<br> | ||
| + | DNA and cells werde mixed softly <br> | ||
| + | Incubation on ice for 20-30 min<br> | ||
| + | Heat shock at 42°C for 40 sek <br> | ||
| + | cells were cooled down on ice for 5-10 min<br> | ||
| + | 900µl sterile 2YT Medium was added<br> | ||
| + | Incubation at 37°C for 60-70 min (shaker)<br> | ||
| + | cells werde plated on 2YT-agar-plates with antibiotics<br> | ||
| + | <br> | ||
| + | Klenow fill in reaction | ||
| + | - 25pmol forward primer | ||
| + | - 25pmol reverse primer | ||
| + | - 0,5 µl Klenow-fragment without exonuclease activity (Fermentas) | ||
| + | - 2µl Klenow Buffer | ||
| + | - 1µl dNTPs | ||
| + | - Add H¬¬2O to a volume of 20µl | ||
| + | program: 94°C for 3min, cool down to 37°C, adition of klenow enzyme, 37°C for 1 hour | ||
Revision as of 10:09, 28 October 2008
 "
"