Team:Freiburg/Project
From 2008.igem.org
m |
|||
| Line 32: | Line 32: | ||
[[Team:Freiburg_Calcium Imaging|Calcium Imaging]] | [[Team:Freiburg_Calcium Imaging|Calcium Imaging]] | ||
<h2>'''Results:'''</h2> | <h2>'''Results:'''</h2> | ||
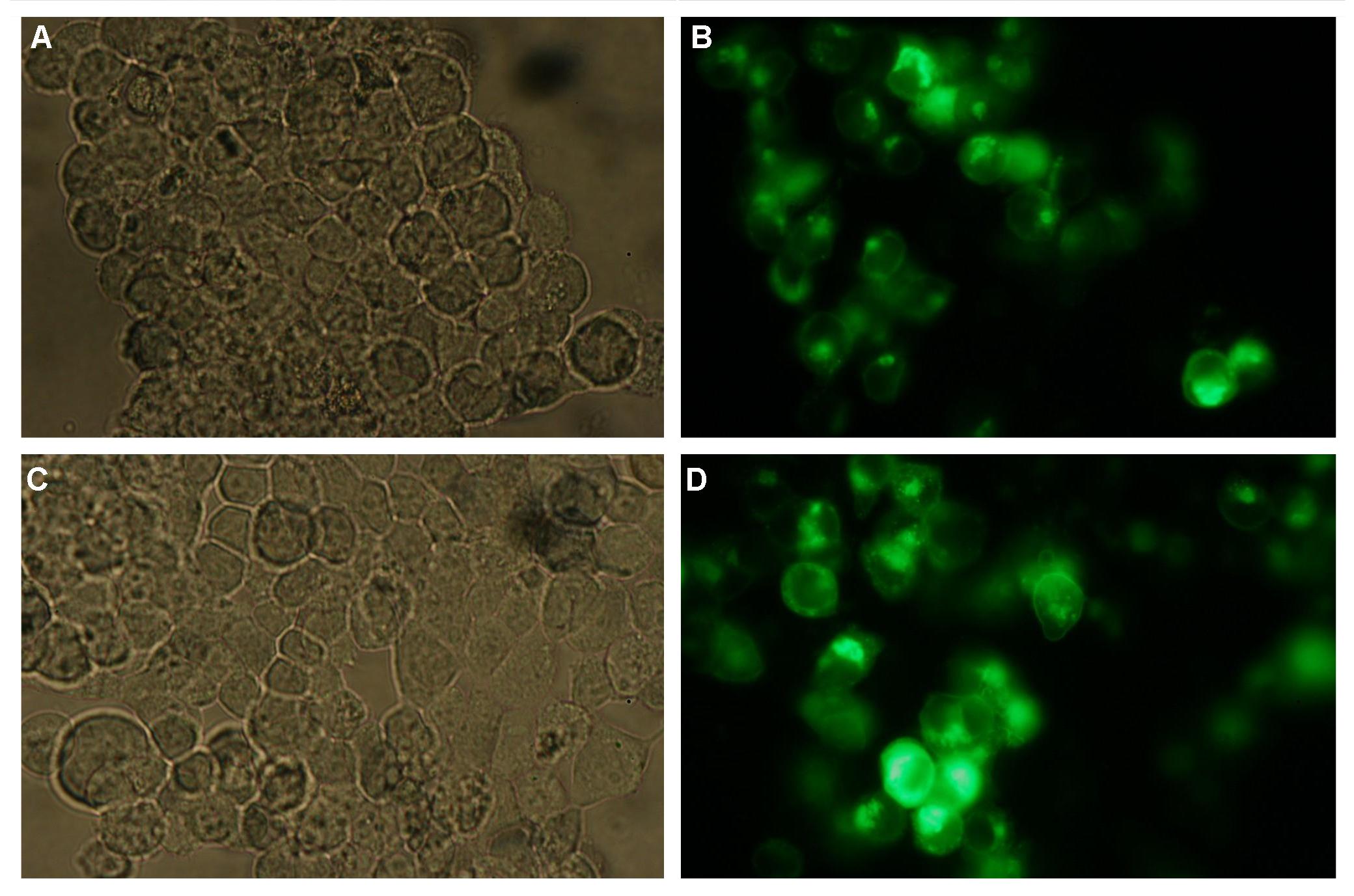
| - | + | All of our constructs (see "Parts") were cloned successfully using our extended pre- and suffix. Transfection of 293T-cells has also been shown to work for most of our fusion proteins; so far, we could even show that at least our constructs with lipocalin FluA as extracellular domaine are integrated into the membrane (Fig.1, details see [[Team:Freiburg Transfection|Transfection]]).<br> | |
[[Image:Freiburg2008_SP_LIPO_GGGS_TM_bla1_YFP_1.jpg|400px]][[Image:Freiburg2008_TV_CMV_YFP___CFP_loeslich.jpg|400px]] | [[Image:Freiburg2008_SP_LIPO_GGGS_TM_bla1_YFP_1.jpg|400px]][[Image:Freiburg2008_TV_CMV_YFP___CFP_loeslich.jpg|400px]] | ||
| - | '''Fig.1:''' left: Transfection of 293T-Cells with one of our membrane-localized constructs (Part Bba_K157032-YFP), <br> right: cytosolic YFP | + | '''Fig.1:''' left: Transfection of 293T-Cells with one of our membrane-localized constructs (Part Bba_K157032-YFP), <br> right: cytosolic YFP<br><br> |
| - | + | All transfections were carried out with part Bba_K157040, one of our composite parts consisting of the transfection vector Bba_J52017 and a CMV-promotor.<br> | |
<h2>'''Discussion:'''</h2> | <h2>'''Discussion:'''</h2> | ||
<h2>'''Literature:'''</h2> | <h2>'''Literature:'''</h2> | ||
| Line 43: | Line 43: | ||
-Tom K. Kerppola: “Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells”, Nat Protoc. 2006;1(3):1278-1286 (doi:10.1038/nprot.2006.201)<br> | -Tom K. Kerppola: “Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells”, Nat Protoc. 2006;1(3):1278-1286 (doi:10.1038/nprot.2006.201)<br> | ||
-Nagai, T. et al. “A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications” J. Biol. Chem. 276, 29188-29194, 2001<br> | -Nagai, T. et al. “A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications” J. Biol. Chem. 276, 29188-29194, 2001<br> | ||
| - | -Roger Y. Tsien et al. „Creating new fluorescent probes for cell biology“, Nature Biotechnology Reviews, Vol. 3, 906-918, 2002 | + | -Roger Y. Tsien et al. „Creating new fluorescent probes for cell biology“, Nature Biotechnology Reviews, Vol. 3, 906-918, 2002<br> |
'''LipocalinFluA:'''<br> | '''LipocalinFluA:'''<br> | ||
-Gerald Beste, Frank S. Schmidt, Thomas Stibora and A. Skerra: “Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold“,Proc. Natl. Acad. Sci. USA Vol. 96, pp. 1898–1903, March 1999 Biochemistry<br> | -Gerald Beste, Frank S. Schmidt, Thomas Stibora and A. Skerra: “Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold“,Proc. Natl. Acad. Sci. USA Vol. 96, pp. 1898–1903, March 1999 Biochemistry<br> | ||
Revision as of 20:30, 28 October 2008
|
Project Report |
_project report
Introduction:This year´s main project is the attempt to create an "artificial receptor-system", featuring extra- and intracellular modules as well as suitable transmembrane regions.
The intracellular domaine of our receptor-device is build by halves of split reporter-proteins that can reassemble and will then produce readable output, e. g. fluorescence.
Each one of these protein-halves is connected to its extracellular domaine by a single-span transmembrane-helix.
The extracellular or detecting domaine consists of a protein or peptide with the ability to bind a certain molecule. Subprojects:DNA-Origami Results:All of our constructs (see "Parts") were cloned successfully using our extended pre- and suffix. Transfection of 293T-cells has also been shown to work for most of our fusion proteins; so far, we could even show that at least our constructs with lipocalin FluA as extracellular domaine are integrated into the membrane (Fig.1, details see Transfection). Discussion:Literature:Split-fluorophores: |
 "
"