Team:UC Berkeley/Assembly
From 2008.igem.org
Contents |
Introduction
Layered assembly is a straight-forward and robust method for combining two or more basic parts into a destination vector. There are three layers (Entry, Assembly and Destination). Both Entry and Assembly layers are standardized for ease of use, while the Destination plasmid can be tailored to a specific experiment.
We propose to create an in-vivo approach to layered assembly by creating cells which stably express excisionase (xis), intergrase (int), ligase (lig), Cre (cuts Xho restriction site) and the BglII and BamHI restriction enzymes.
Entry Layer
The Entry plasmid contains a Spectinomycin antibiotic resistance marker and EcoRI, BglII and BamHI restriction sites. All basic parts are cloned into the entry vector. The basic part is flanked by attR1 and attr2 recombination sites.
Since the entry plasmid attR1 and attR2 recombination sites, the basic part can easily be transferred into one of six different assembly vectors using the Gateway cloning scheme described here [[1]].
Assembly Layer
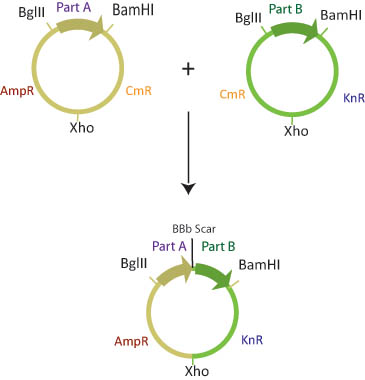
The six assembly plasmids contain two of three different antibiotic resistance markers (kanamycin, ampilicin and Chloramphenicol) separated by a XhoI restriction site.
The assembly plasmids are transformed into cells that are engineered to either methylate BamHI or BglII restriction sites. Part A is transformed into a "lefty" cell that is methylated on BglII restriction sites while Part B is transformed into a "righty" cell that is methylated on BamHI restriction sites.
Lefty cells are engineered to stably express ligase and the BglII restrictions enzyme and are methylated on BglII cut sites. Righty cells are engineered to stably express XhoI and BamHI restriction enzymes and are methylated on BamHI cut sites. These cells are co-transformed with a plasmid containing our lysis device.
In a single eppendorf tube, lefty and righty cells containing basic part DNA are lysed using our lysis device. Click here for more information.[[2]]
The basic part DNA, along with the restriction enzymes are released into solution. Since restriction enzymes will not cut methylated DNA, the restriction site BglII site in the lefty cell and the BamHI restriction site in the righty cell are blocked from digestion. The BamHI site in lefty and the BglII site in lefty, and the Xho sites in both plasmids will be cut. Ligase will join the xho sites and the BglII/BamHI complimentary sticky ends.
The choice of antibiotic resistance markers in the assembly plasmids is predetermined such that once the two plasmids recombine, the new plasmid will have a combination of markers from the two assembly vectors. Although there are four possible products of this reaction, only one will have both of the correct antibiotic resistance markers.
The assembly reaction can be repeated to combine a number of basic parts in a desired order.
Once the composite part is complete, it is transferred to a destination plasmid.
Destination Layer
Transfer of the composite part to the Destination plasmid using another Gateway cloning reacion [[3]].
The Destination plasmid is tailored to a specific experiment or assay. This allows a single composite part to be transfered to several different Destination plasmids for further experimentation or characterization.
 "
"