Team:Brown/Project/Testing
From 2008.igem.org
(Difference between revisions)
| Line 14: | Line 14: | ||
==Revisions to the First Apparatus== | ==Revisions to the First Apparatus== | ||
| - | * | + | * Our first apparatus presented many complications. First and foremost was the sensitivity factor. We calculated the amount of E. coli bacteria cells expected in each sample and the corresponding net ionic content. These values cannot be accurately measured with a standard voltmeter. |
| - | [[Image:Brown lego appr.jpg|right|thumb|400px|Apparatus | + | * In addition, the electrodes of the first apparatus did not remain stationary and were difficult to manage. They were made of Copper alloy and multi-stranded. The copper was most likely performing redox chemistry. The DNA and other charged particles released from the cells collected on the wires throughout the experiment. |
| - | [[Image:Brown daq and cb.jpg|right|thumb|400px| | + | |
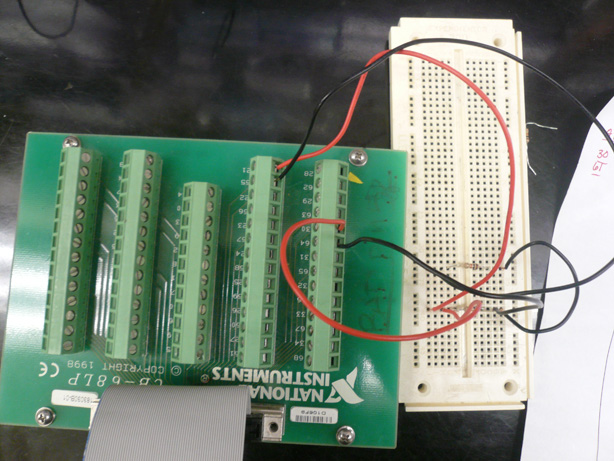
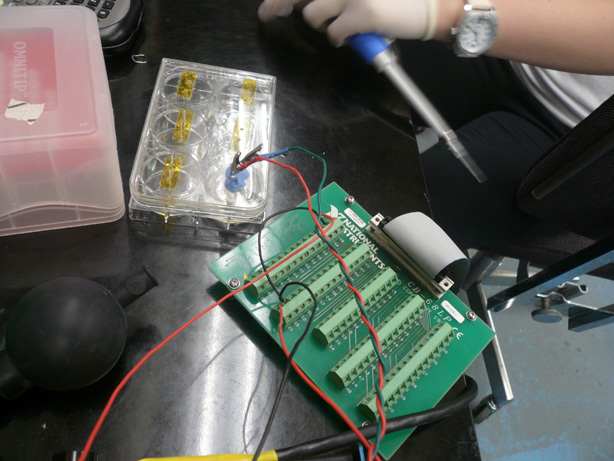
| - | [[Image:Brown daq and mold.jpg|right|thumb|400px|Apparatus | + | * Our new apparatus utilized a Data Aquistion Card and the LabView Program. Team Toxipop created an electrical circuit within LabView. |
| + | * The new apparatus also consisted of a six-well plate, PDMS Mold, and Platinum Wires. | ||
| + | [[Image:Brown lego appr.jpg|right|thumb|400px|New Apparatus]] | ||
| + | [[Image:Brown daq and cb.jpg|right|thumb|400px|NewApparatus]] | ||
| + | [[Image:Brown daq and mold.jpg|right|thumb|400px|New Apparatus]] | ||
Revision as of 03:04, 29 October 2008
|
Resistance
Our First Apparatus
Revisions to the First Apparatus
|
 "
"