Team:ETH Zurich/Project/Medal Relevant
From 2008.igem.org
m |
m |
||
| Line 13: | Line 13: | ||
<!-- PUT THE PAGE CONTENT AFTER THIS LINE. THANKS :) --> | <!-- PUT THE PAGE CONTENT AFTER THIS LINE. THANKS :) --> | ||
== Medal Relevant Issues == | == Medal Relevant Issues == | ||
| + | === Bronze Relevant === | ||
| + | ====Register the team, have a great summer, and have fun attending the Jamboree==== | ||
| + | ====Successfully complete and submit a Project Summary form==== | ||
| + | ====Create and share a Description of the team's project via the iGEM wiki==== | ||
| + | ====Present a Poster and Talk at the iGEM Jamboree==== | ||
| + | ====Enter information detailing at least one new standard BioBrick Part or Device in the Registry of Parts==== | ||
| + | ====Submit DNA for at least one new BioBrick Part or Device to the Registry of Parts==== | ||
=== Silver Relevant === | === Silver Relevant === | ||
| + | ====Demonstrate that at least one new BioBrick Part or Device of your own design and construction works as expected==== | ||
| + | ====Characterize the operation of at least one new BioBrick Part or Device and enter this information on the Parts or Device page via the Registry of Parts==== | ||
| + | |||
=== Gold Relevant === | === Gold Relevant === | ||
Revision as of 18:00, 27 October 2008
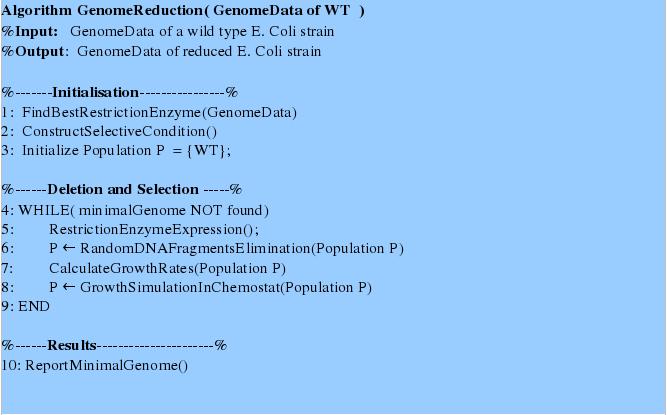
Medal Relevant IssuesBronze RelevantRegister the team, have a great summer, and have fun attending the JamboreeSuccessfully complete and submit a Project Summary formPresent a Poster and Talk at the iGEM JamboreeEnter information detailing at least one new standard BioBrick Part or Device in the Registry of PartsSubmit DNA for at least one new BioBrick Part or Device to the Registry of PartsSilver RelevantDemonstrate that at least one new BioBrick Part or Device of your own design and construction works as expectedCharacterize the operation of at least one new BioBrick Part or Device and enter this information on the Parts or Device page via the Registry of PartsGold RelevantAnalysis, modeling, and simulation of BioBrick Parts or DevicesWe propose a novel method of random gene deletion and chemostat-based selection of species with a reduced genome. For this we provide an algorithm described below. Modeling FrameworkThis algorithm requires a modeling framework consisting of four main parts:
Detailed description of the Modeling Framework
Summary of the Algorithm and Interplay of Frameworks ComponetsFirst tree steps initialize and prepare the system for gene deletions and growth simulations. In the first step the genome data are analyzed in order to find a most suitable restriction enzyme for random fragments deletion using the “Restriction Enzyme Analysis”- procedure. For the second step the state-of-the art model is adjusted by introducing a selective pressure due to the genome size. Thirdly, initial population consists of one type, namely wildtype. The next steps of the algorithm perform in depth genome fragments deletion and growth simulation. First, we simulate the restriction enzyme expression and the consecutive population decline and occurrence of new mutants with reduced genome, which growth rates can be predicted using Flux Balance Analysis. For these simulations we used the framework parts: “Switch generator” and “Flux Balance Analysis on a Genome Scale Model “. Secondly, the growth simulations are performed using a chemostat model and the distribution of different mutant types after the growth type are obtained. This simulation continues until no better mutants can be generated. Eventually, the genome data of the fastest growing reduced genome mutant can be returned.
|
 "
"